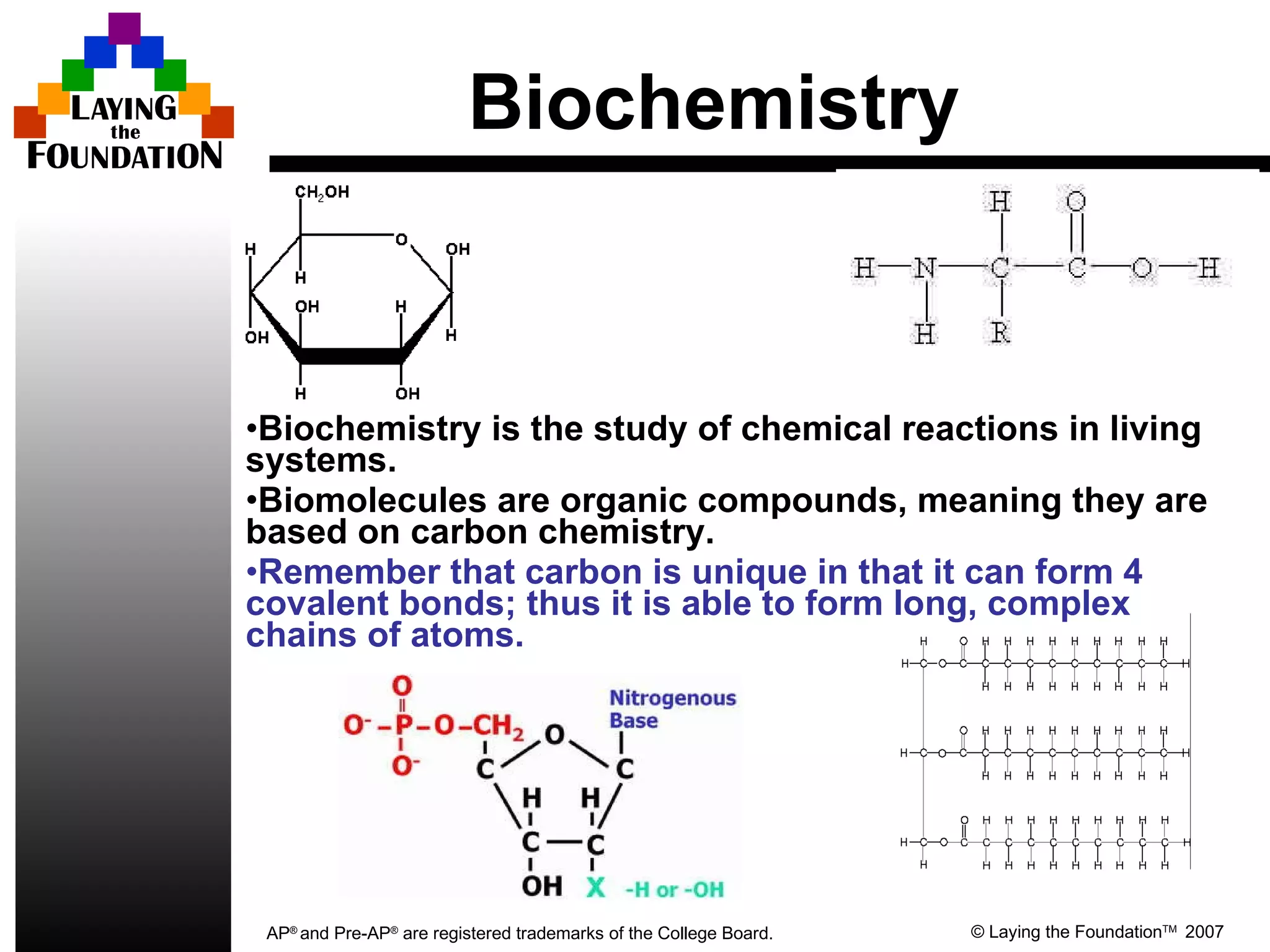
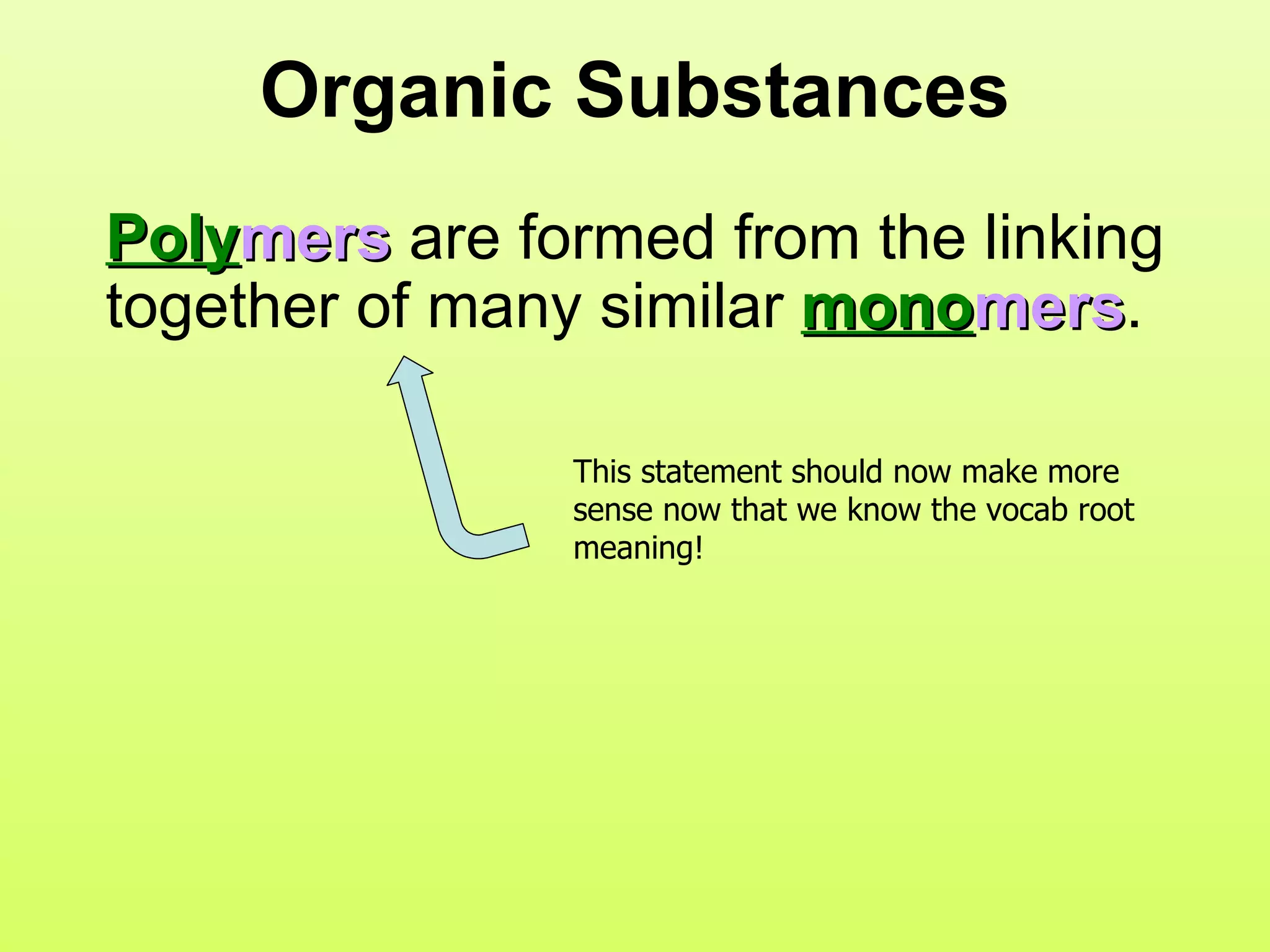
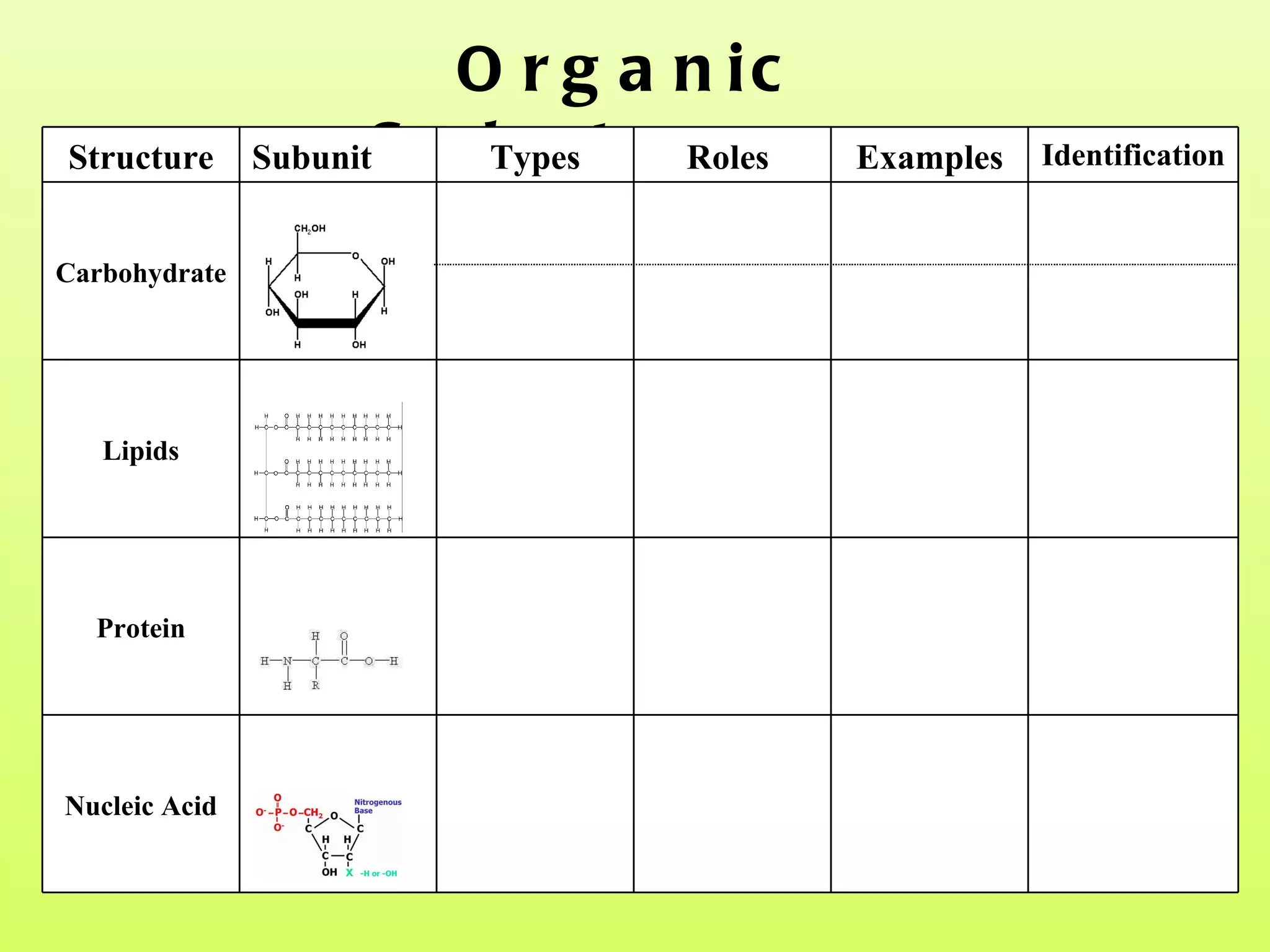
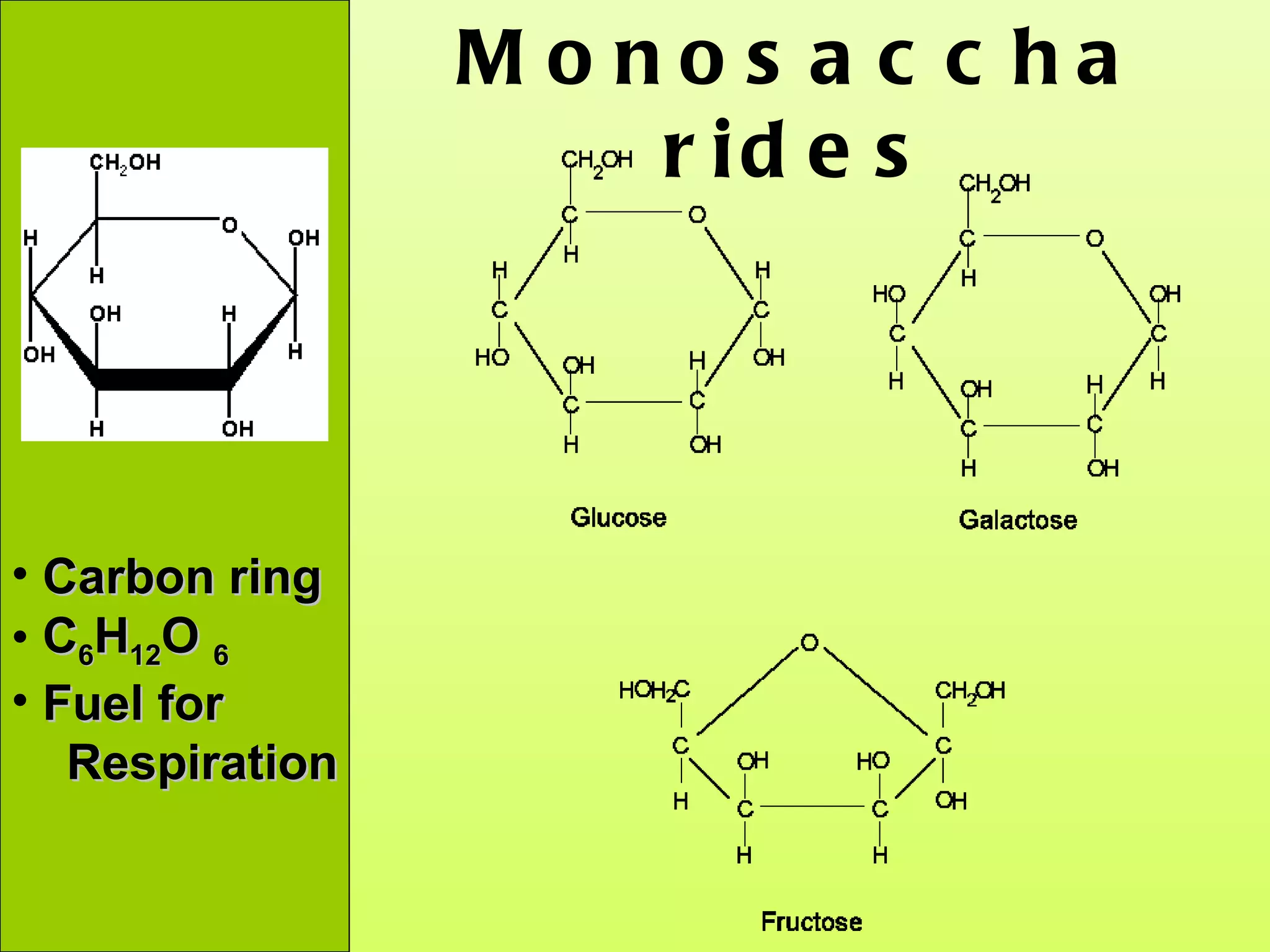
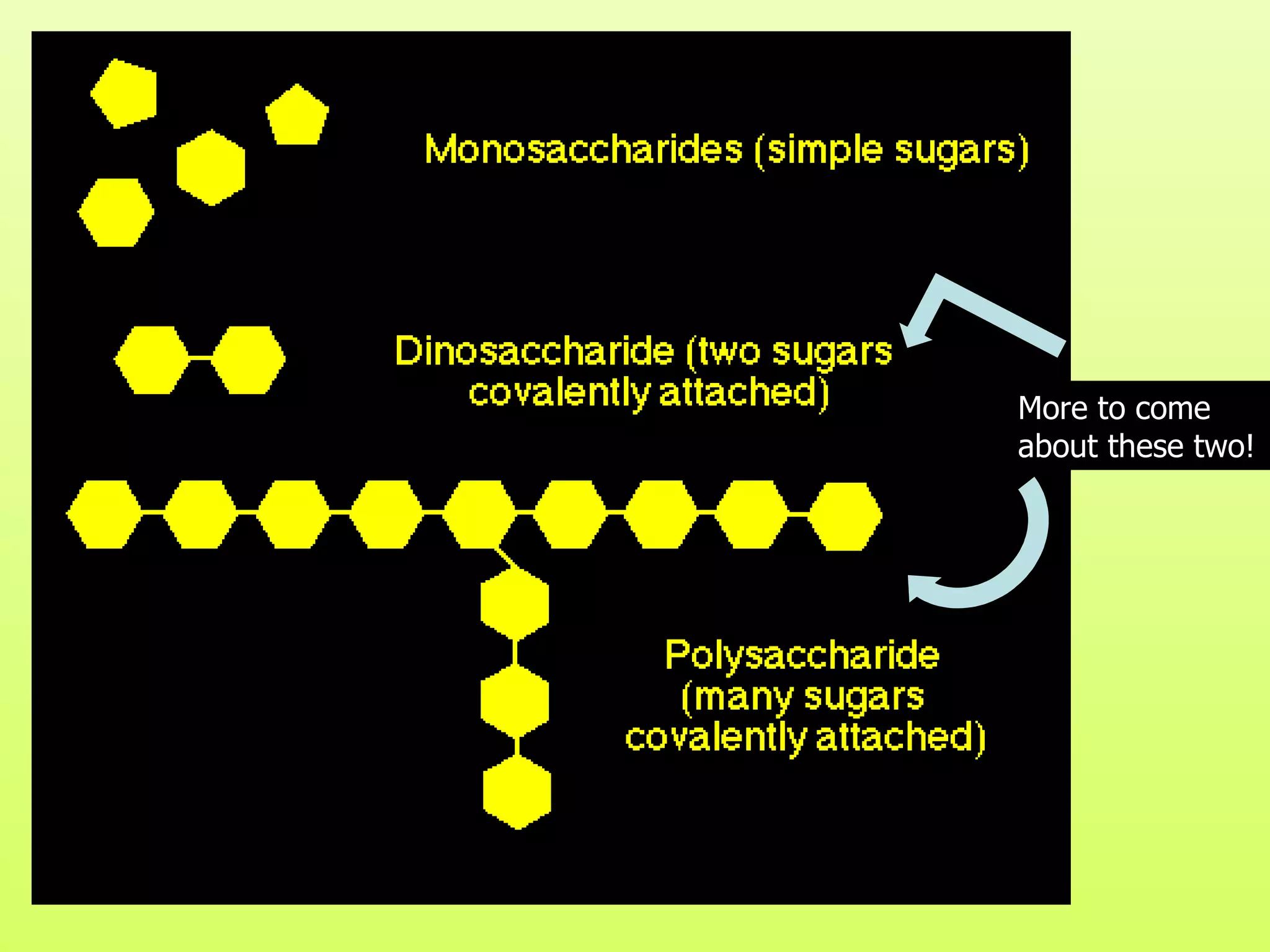
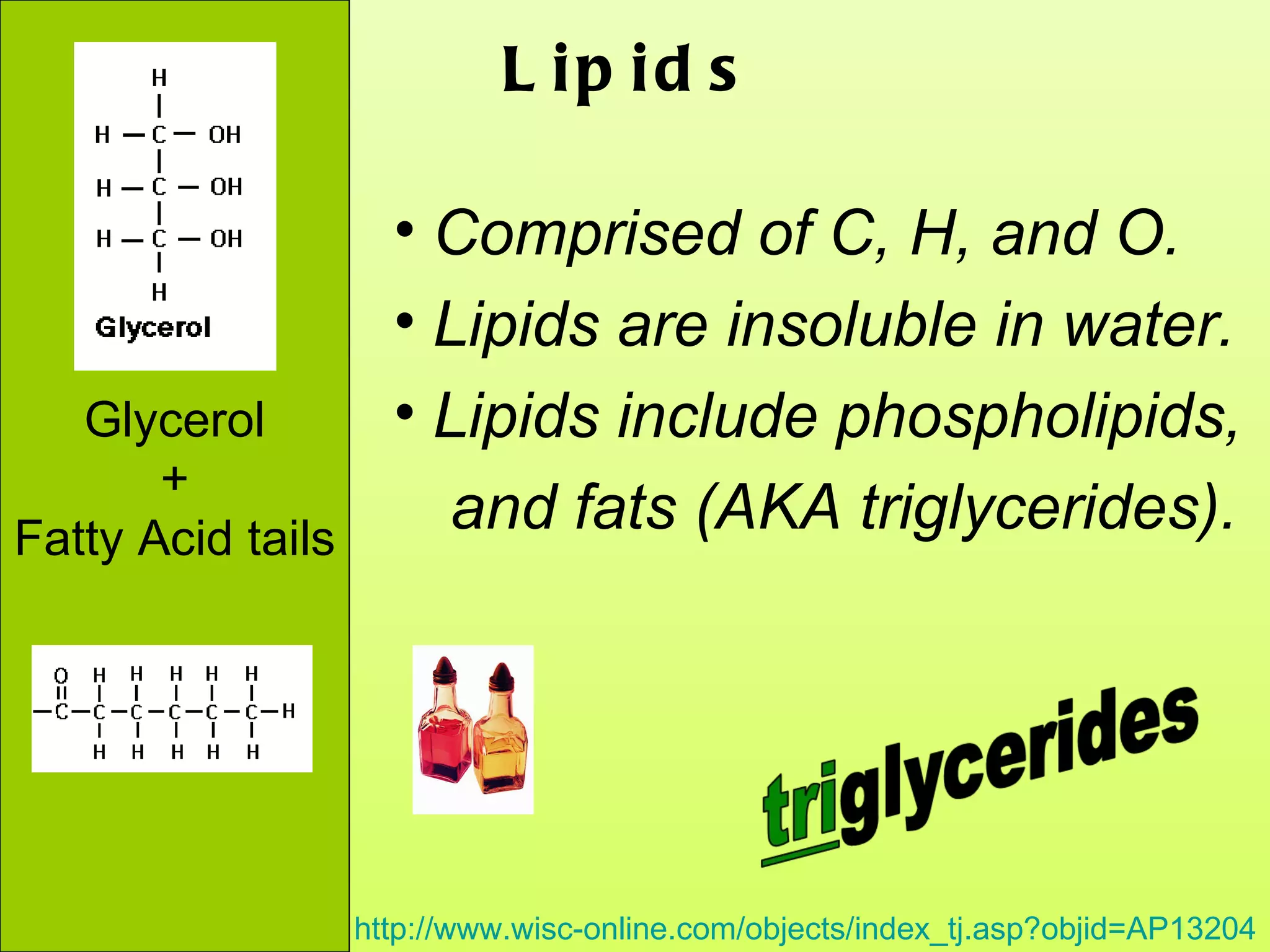
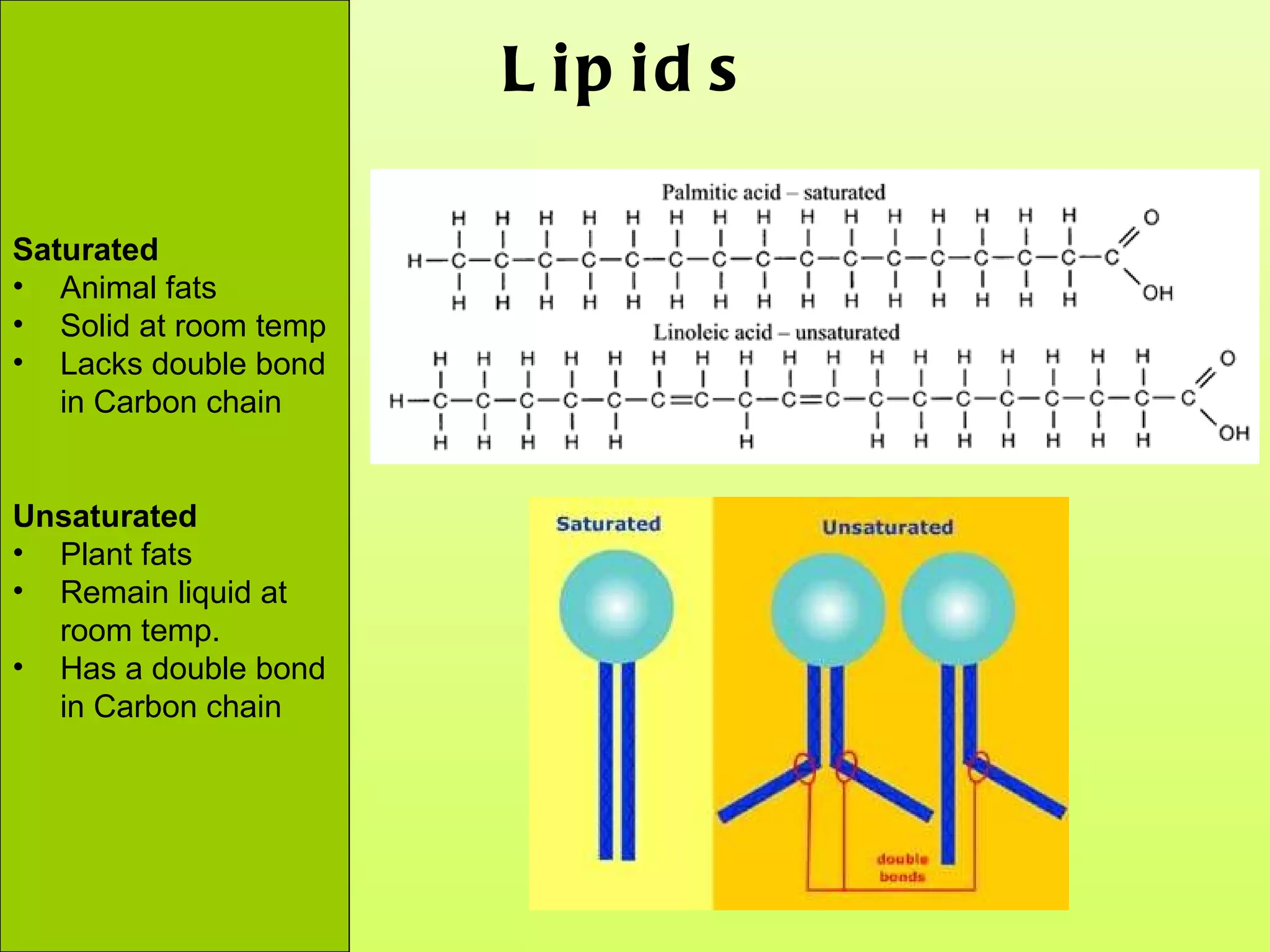
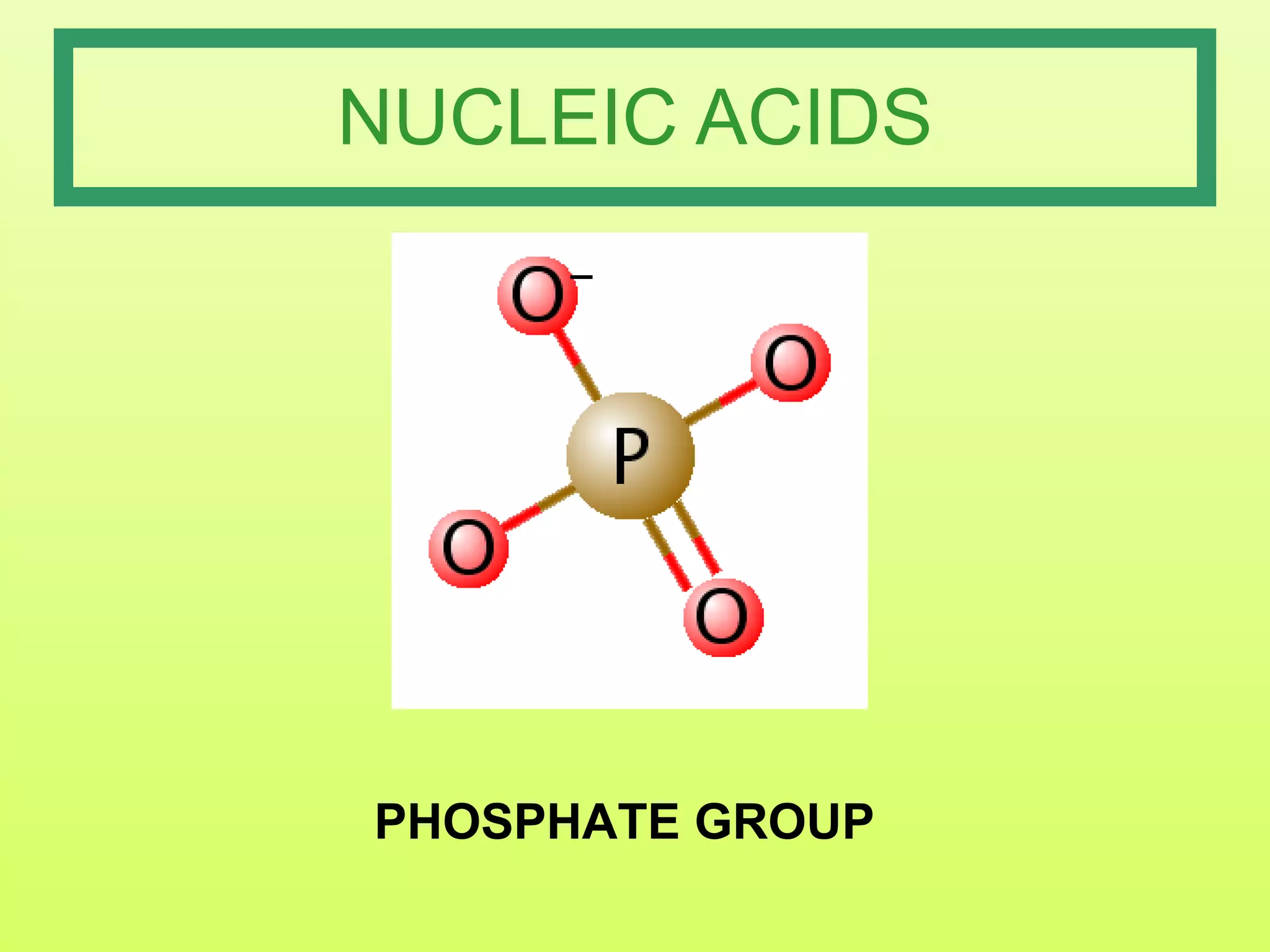
The document provides an introduction to macromolecules (biomolecules) that make up living things. It discusses the four main types of organic macromolecules: carbohydrates, lipids, proteins, and nucleic acids. It describes the monomers (single units) that combine to form polymers (long chains) of each macromolecule, including examples like glucose and fatty acids.