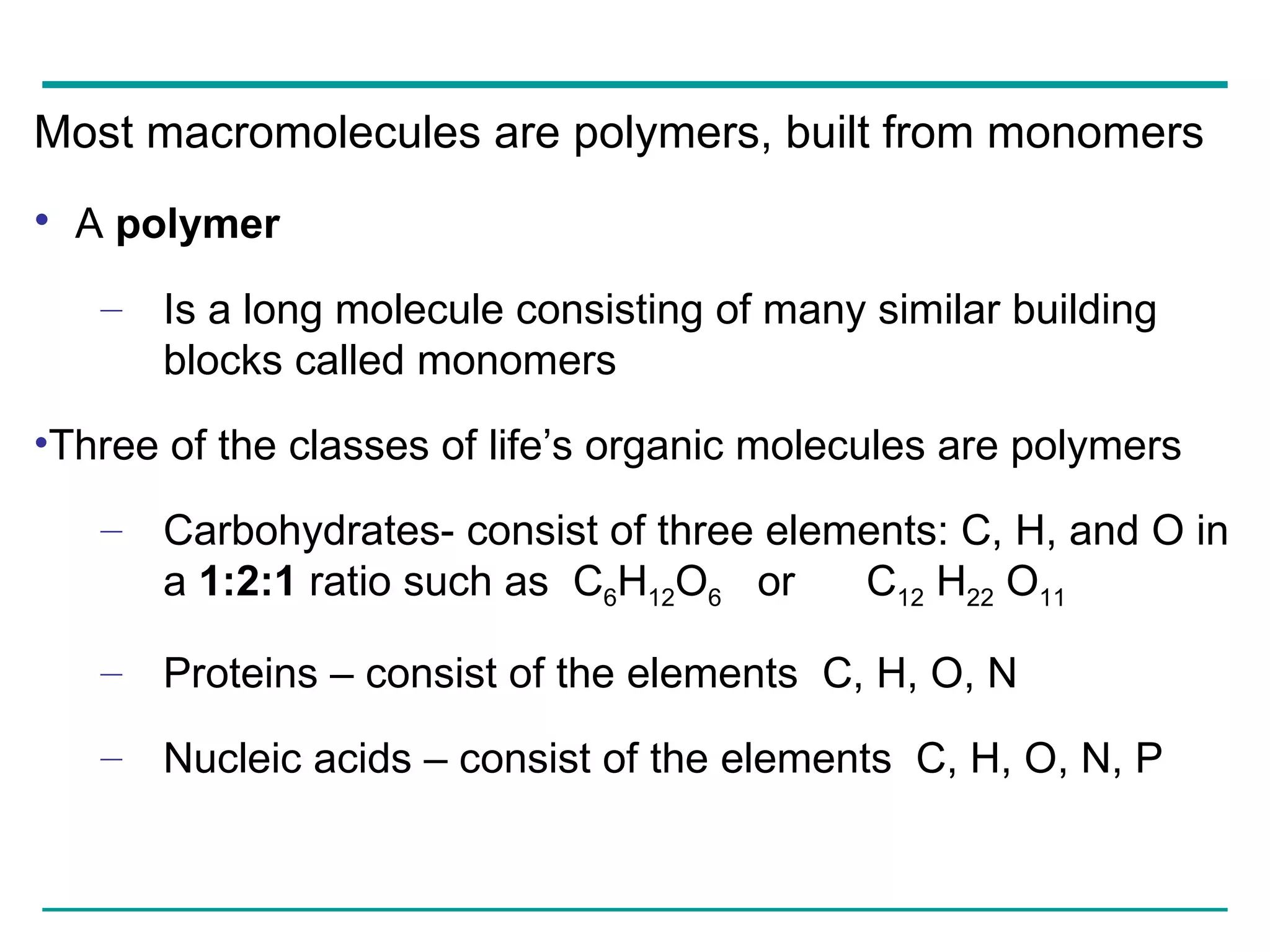
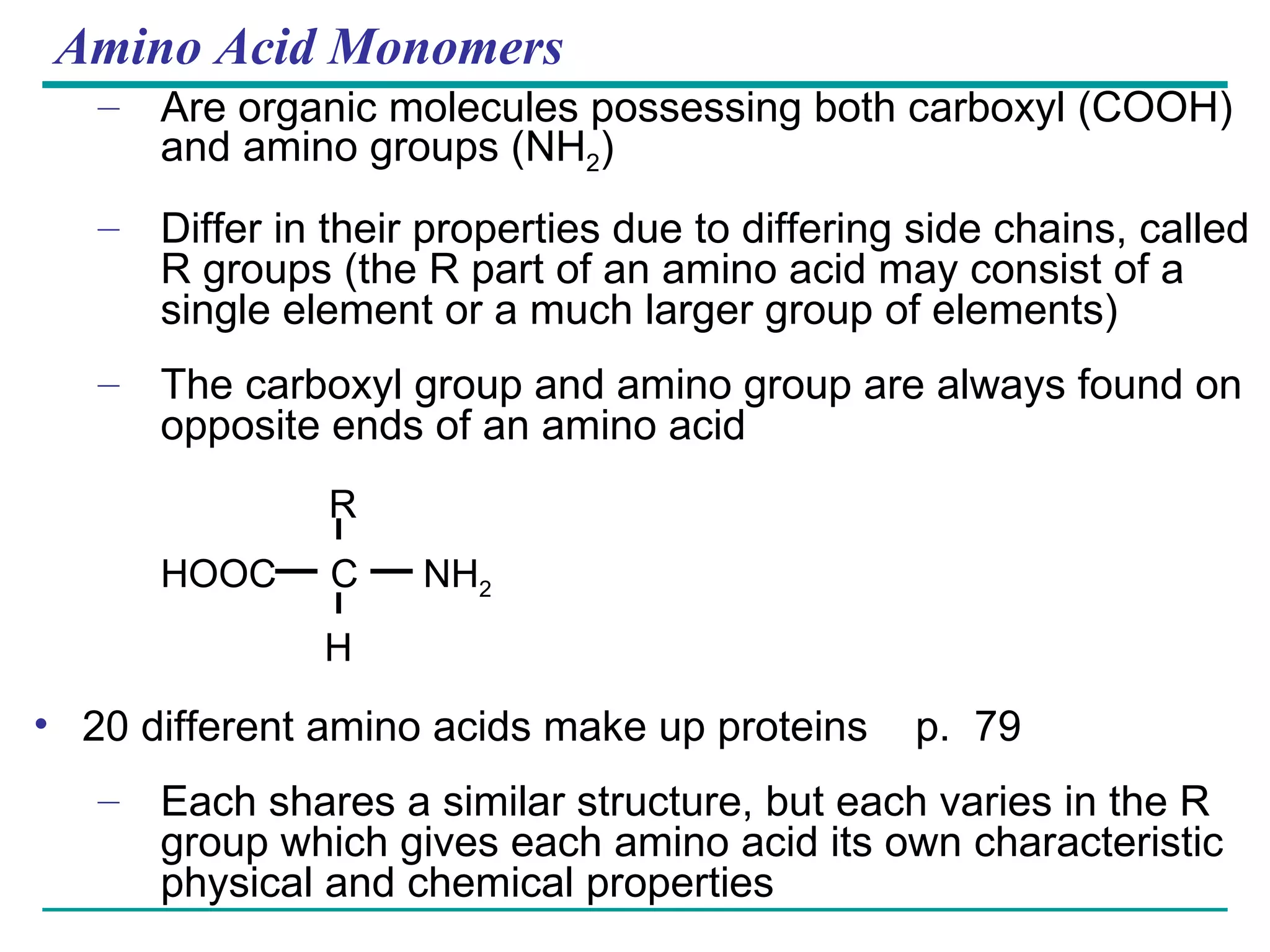
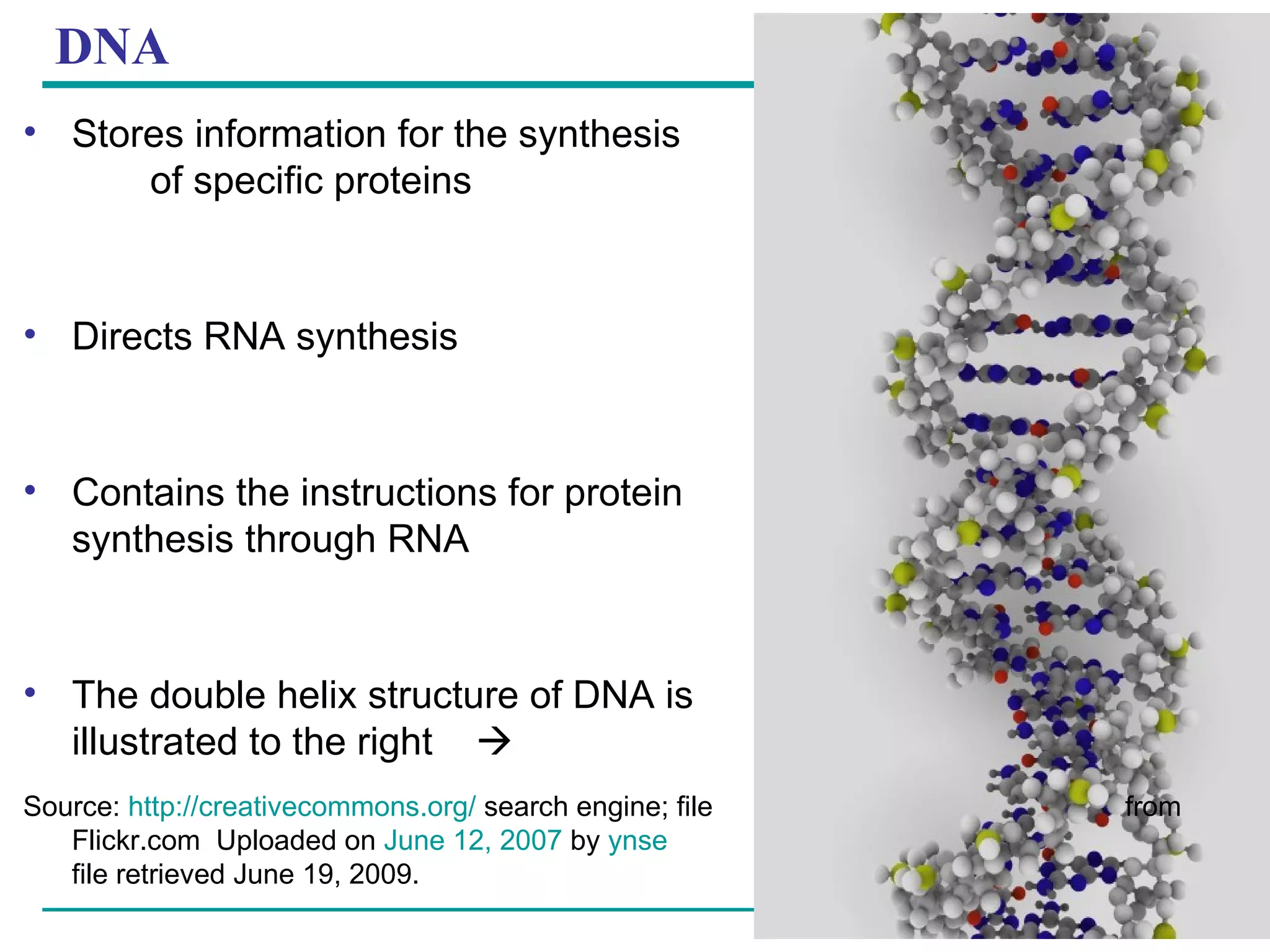
The document summarizes the four major macromolecules - carbohydrates, lipids, proteins, and nucleic acids. It discusses that macromolecules are composed of smaller molecules called monomers, and three of the four classes (carbohydrates, proteins, nucleic acids) are polymers of these monomers. The document also provides examples of monomers that make up each macromolecule type, such as monosaccharides, amino acids, and nucleotides, and describes some of the key functions of each macromolecule class.