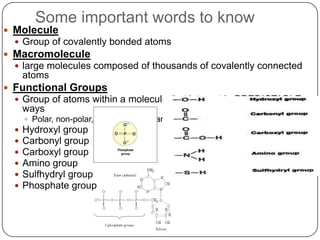
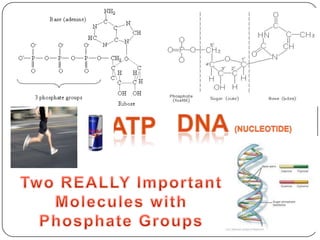
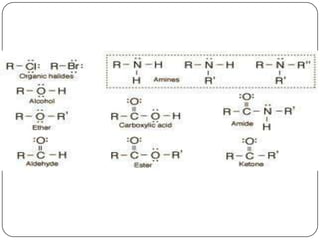
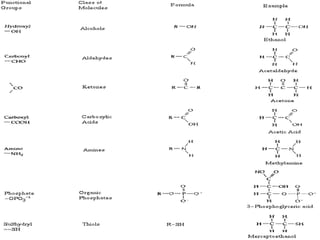
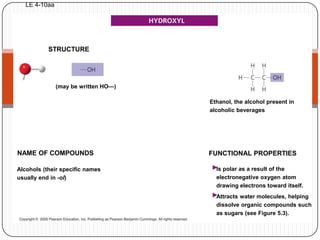
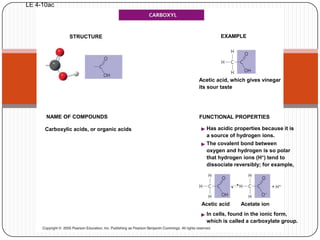
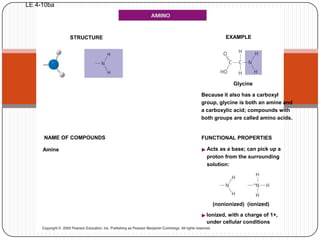
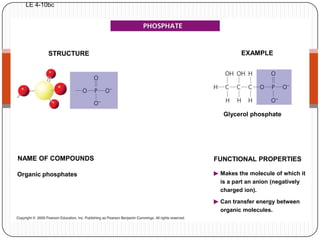
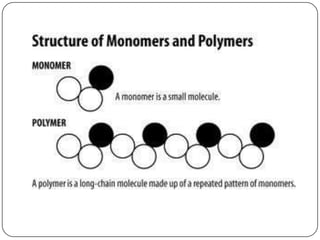
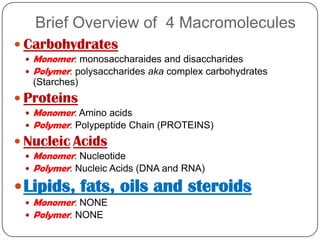
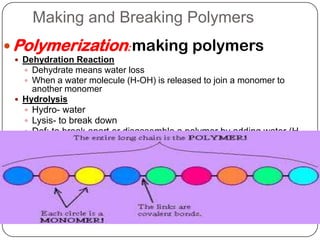
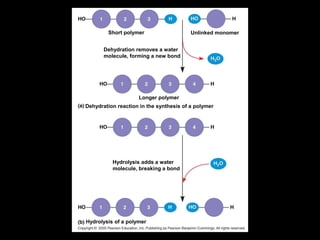
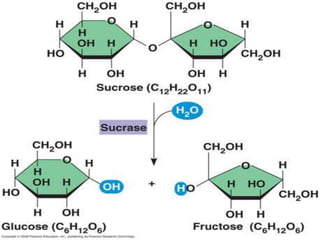
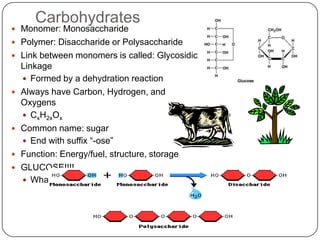
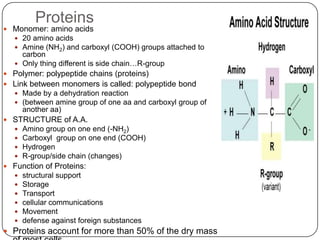
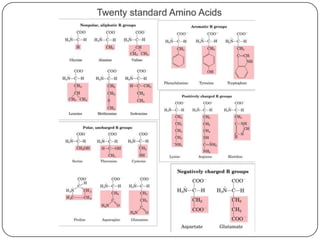
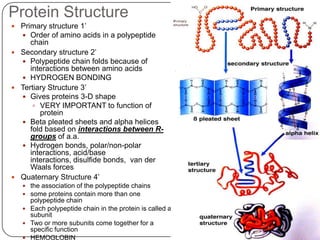
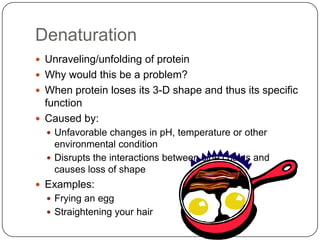
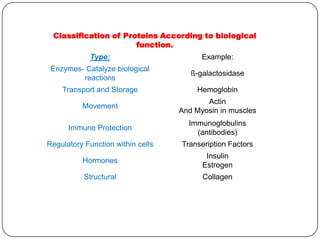
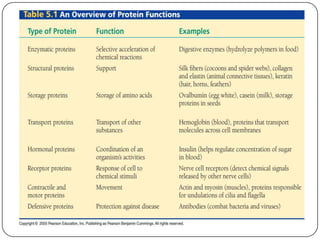
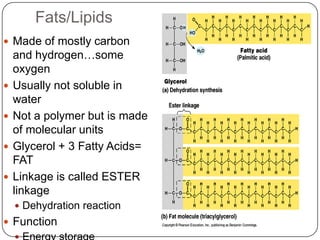
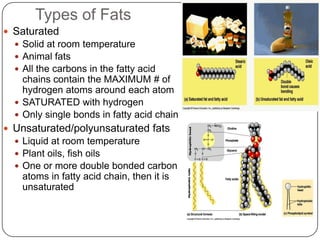
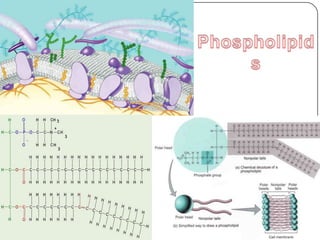
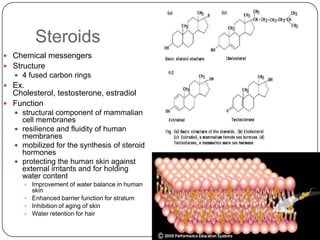
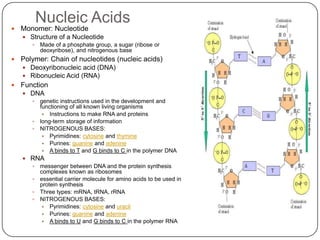
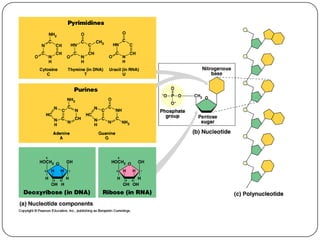
The document discusses the four major macromolecules that make up living organisms: carbohydrates, proteins, lipids, and nucleic acids. It describes the monomers (sugars, amino acids, fatty acids, nucleotides) that polymerize to form each macromolecule, and their structures, functions, and important examples like glucose, glycogen, amino acids, DNA, RNA, glycerol, and fatty acids. It also covers general concepts like dehydration synthesis, hydrolysis, and protein structure levels from primary to quaternary.