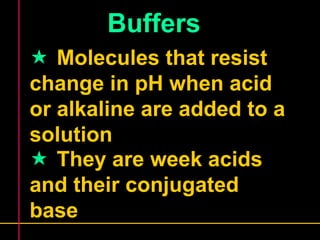
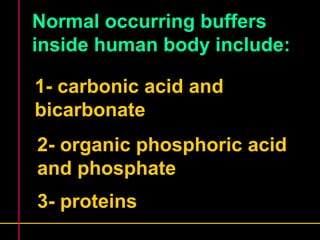
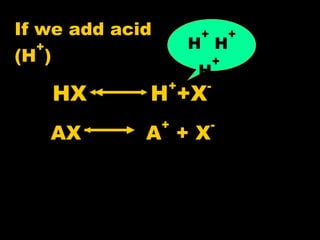
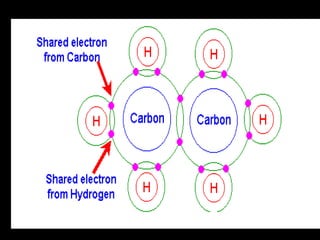
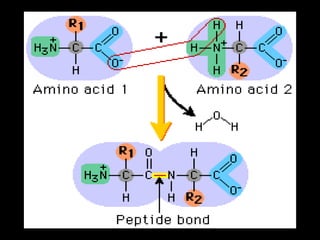
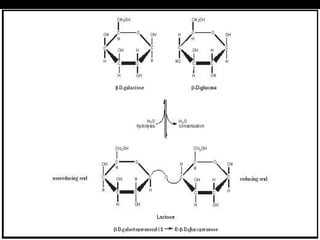
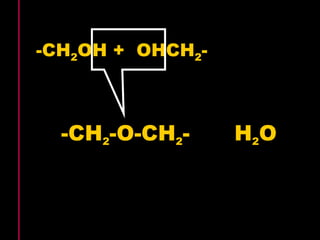
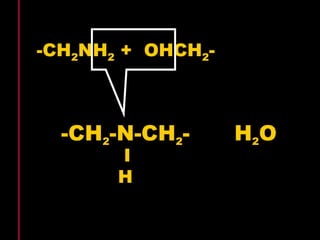
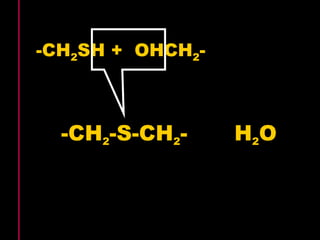
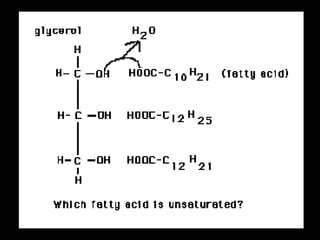
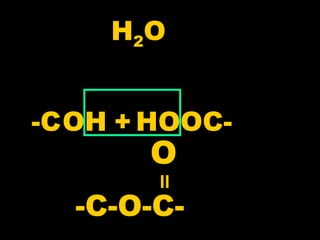
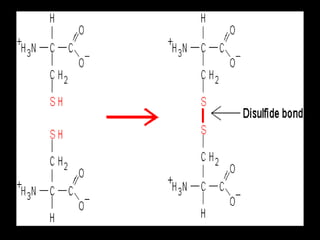
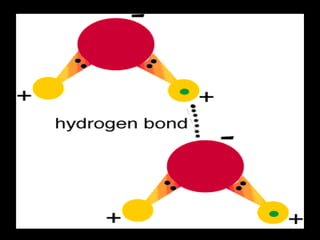
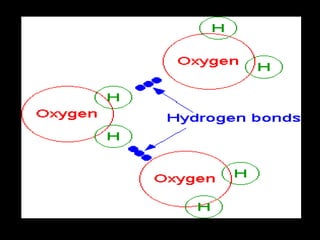
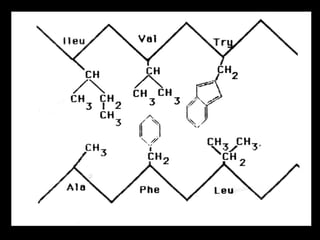
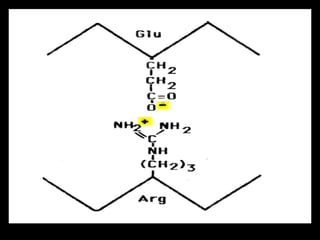
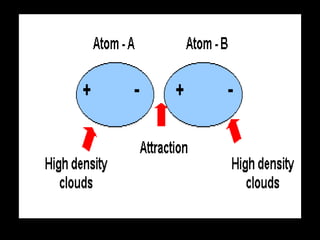
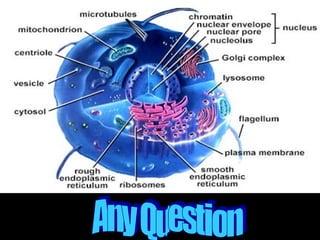
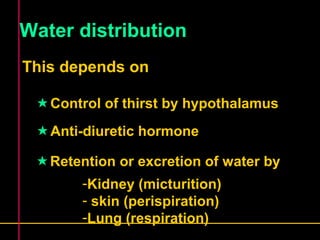
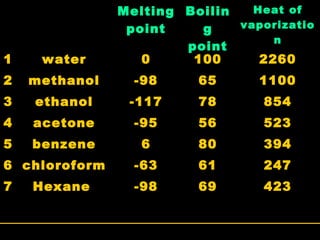
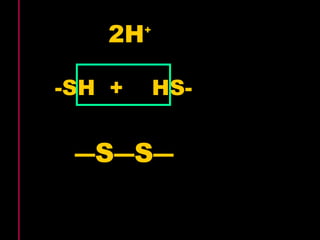Biochemistry is the study of biomolecules in living organisms and their chemical reactions. It includes cell biology, molecular biology, and molecular genetics. The goals of biochemistry are to describe and explain all chemical processes in cells on a molecular level and to understand the origins of life. Biochemistry is essential to medicine, as many diagnostic techniques and drugs are developed based on biochemical discoveries and processes. The basic units of life, cells, can be either eukaryotic or prokaryotic. Biomolecules include carbohydrates, proteins, lipids, nucleic acids, minerals, vitamins, and water, which are held together by covalent and non-covalent bonds.













![pH
Potential hydrogen
Hydrogen ion concentration
Is –log [H+]
pH scale 1—14
7 neutral
Normal pH 7.35 – 7.45](https://image.slidesharecdn.com/introductiontobiochemistry-141211141336-conversion-gate01/85/Introduction-to-biochemistry-21-320.jpg)