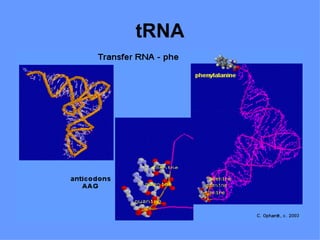
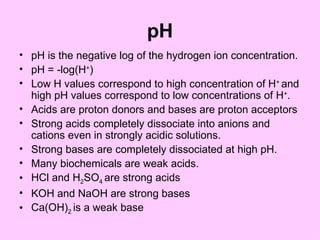
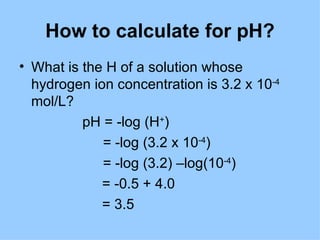
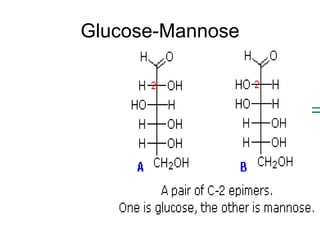
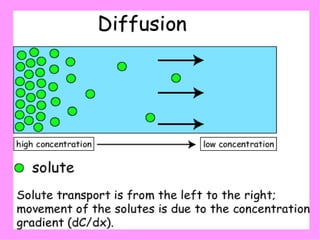
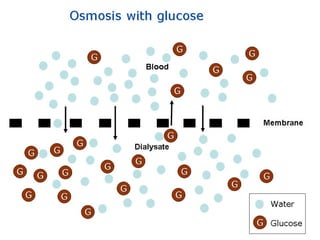
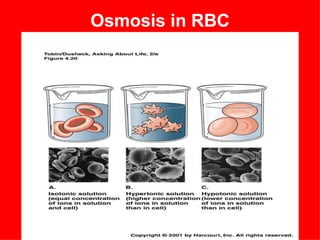
1. The document provides an introduction to biochemistry including defining it as the science concerned with chemical basis of life and chemical constituents of living cells.
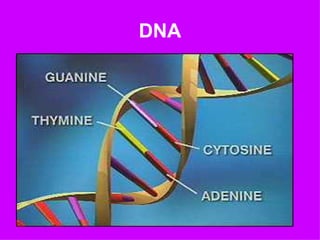
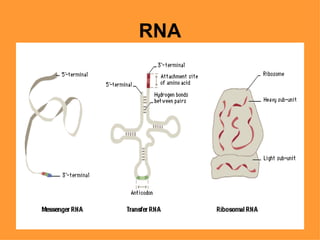
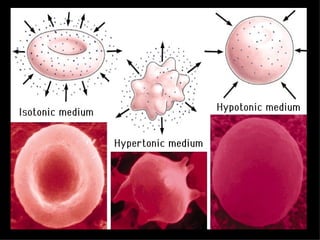
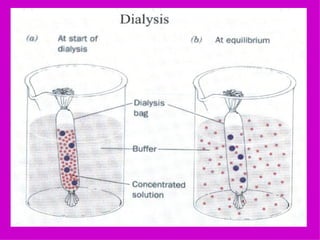
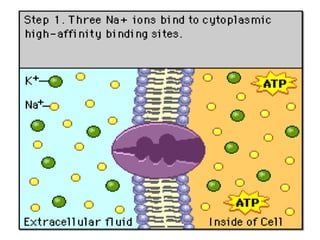
2. It describes the key components of living matter including water, inorganic substances, and organic biomolecules.
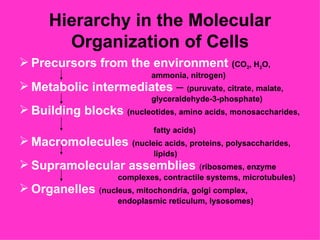
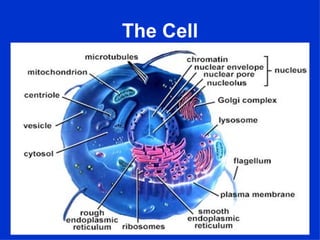
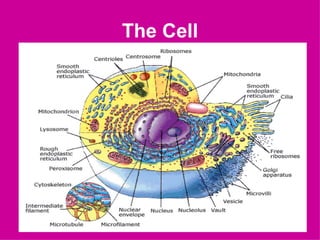
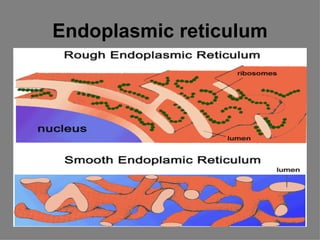
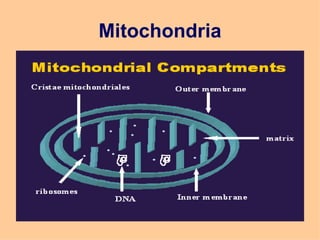
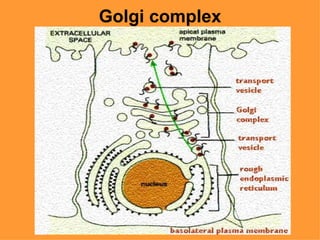
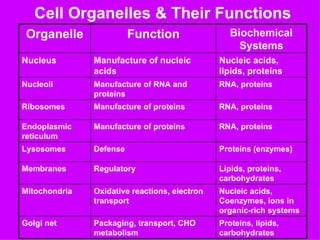
3. The key cellular organelles such as nucleus, mitochondria, endoplasmic reticulum, Golgi complex, lysosomes, and their functions are outlined.