This document summarizes key concepts from a chapter on chemistry in biology, including:
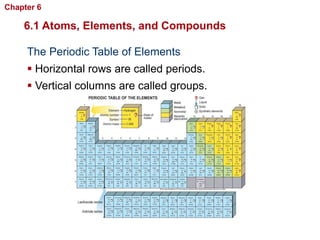
- Elements are pure substances that cannot be broken down further, and compounds are formed when elements combine.
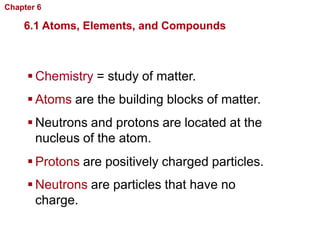
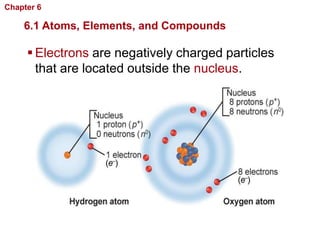
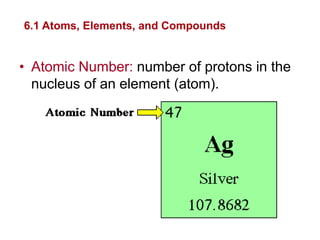
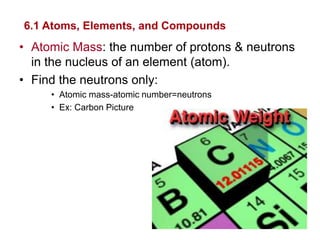
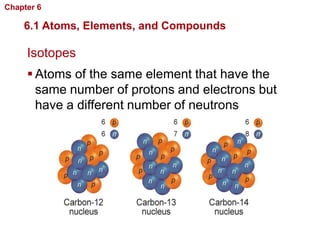
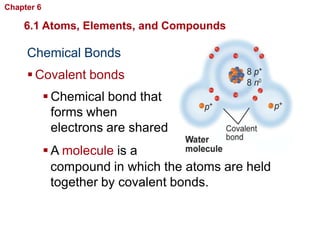
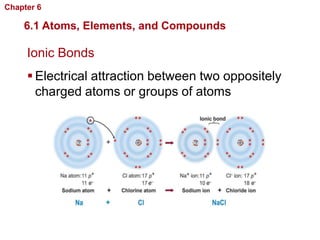
- Atoms are made of protons, neutrons, and electrons. Chemical bonds like covalent and ionic bonds form between atoms.
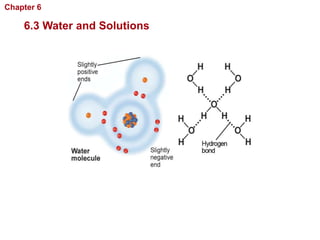
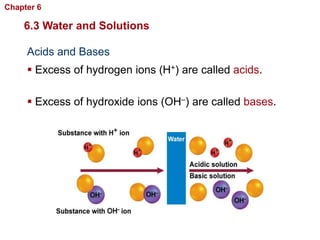
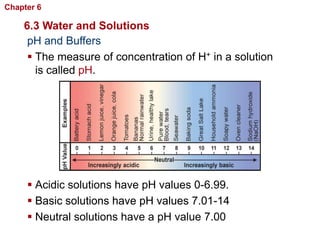
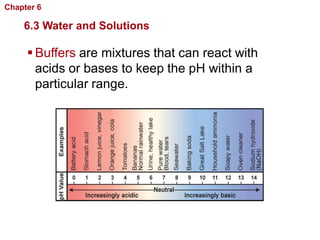
- Water has unique properties like polarity that allow it to dissolve many other substances.
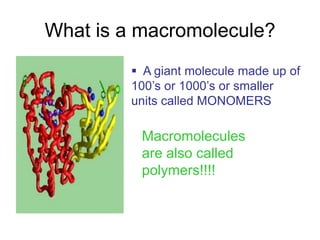
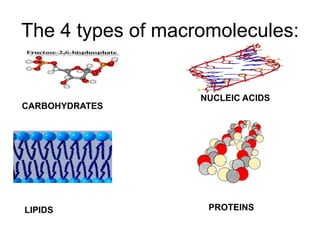
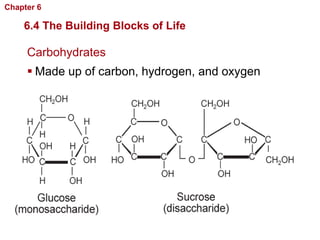
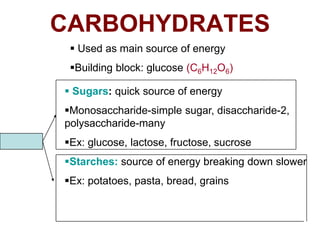
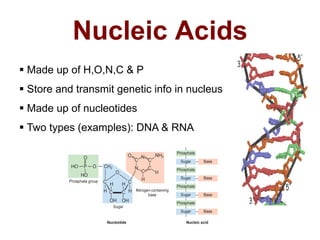
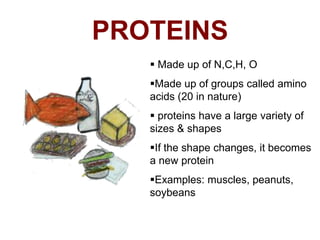
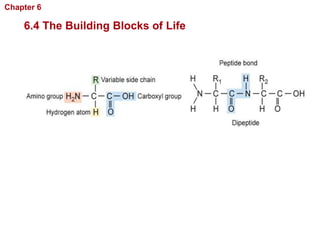
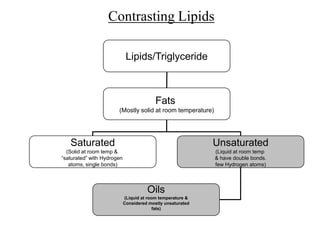
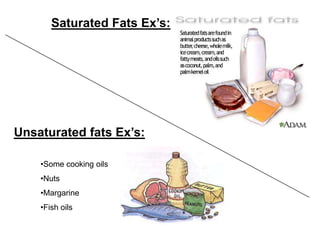
- The four main macromolecules that make up living things are carbohydrates, lipids, proteins, and nucleic acids.
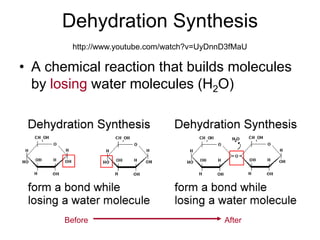
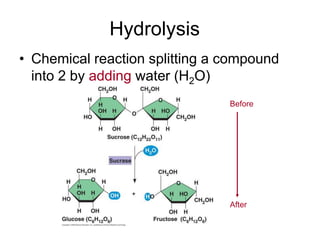
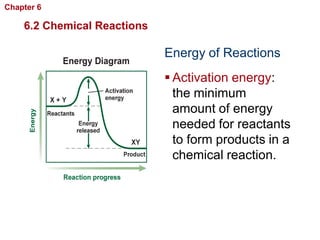
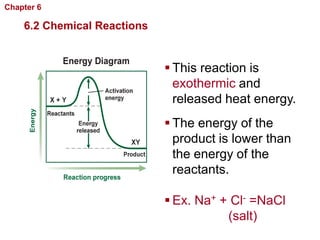
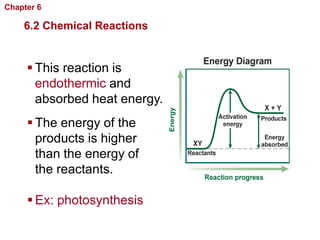
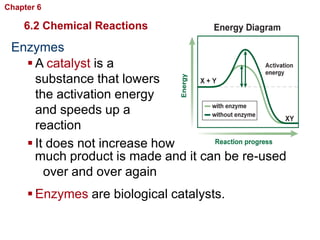
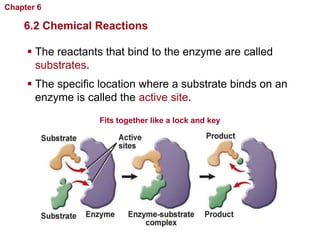
- Chemical reactions involve reactants forming products, and can be exothermic or endothermic. Enzymes act as biological catalysts in reactions.