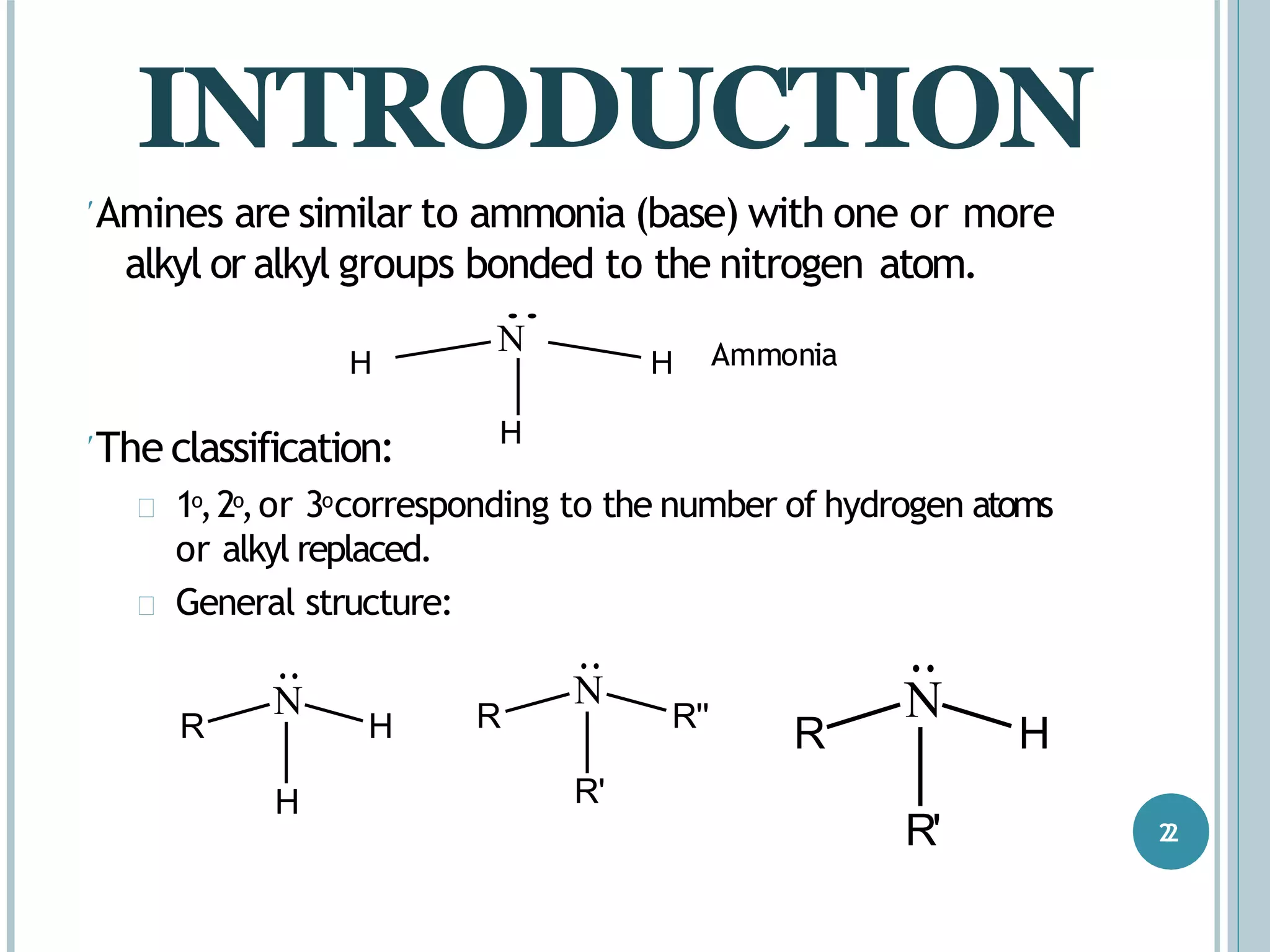
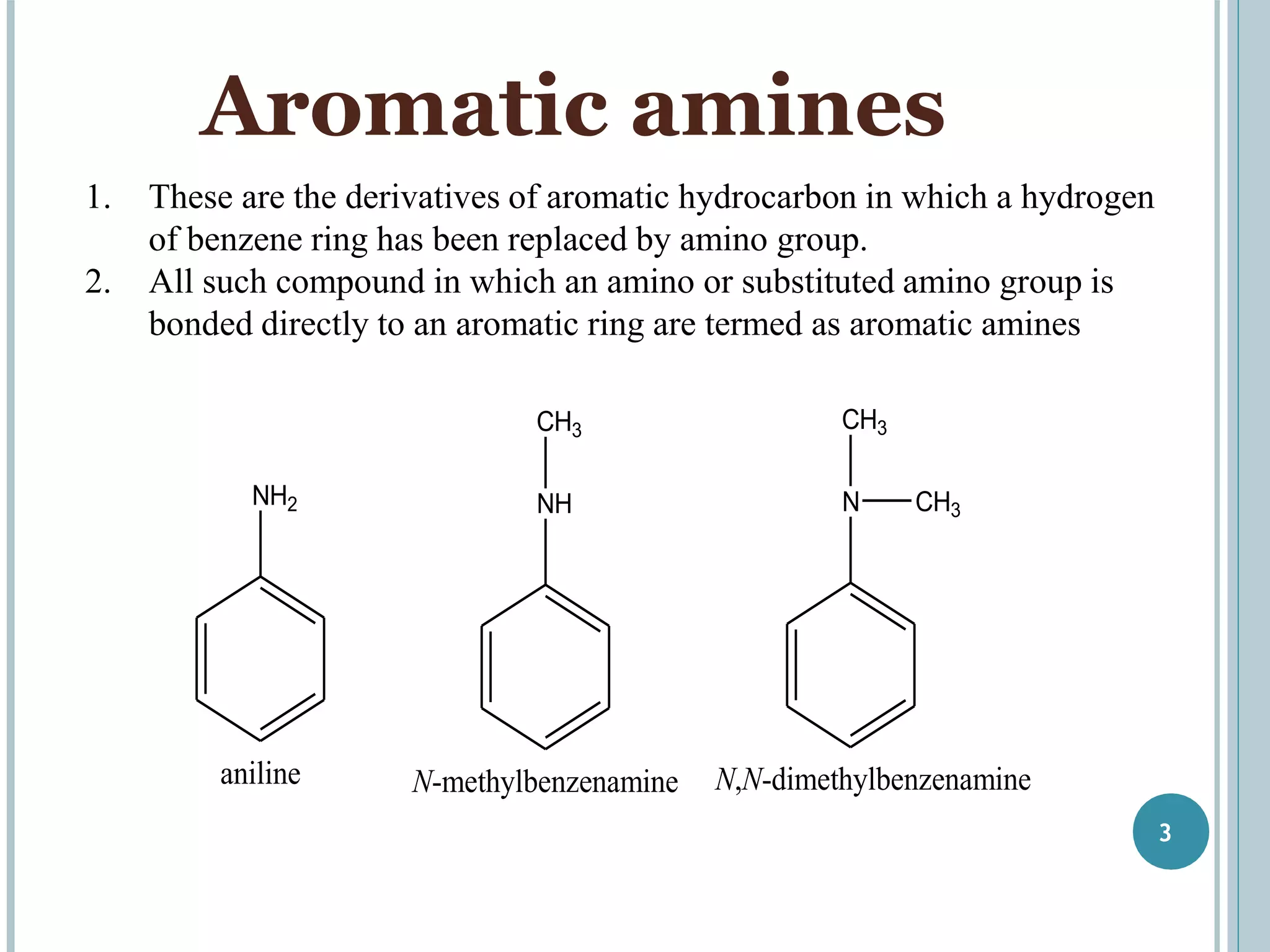
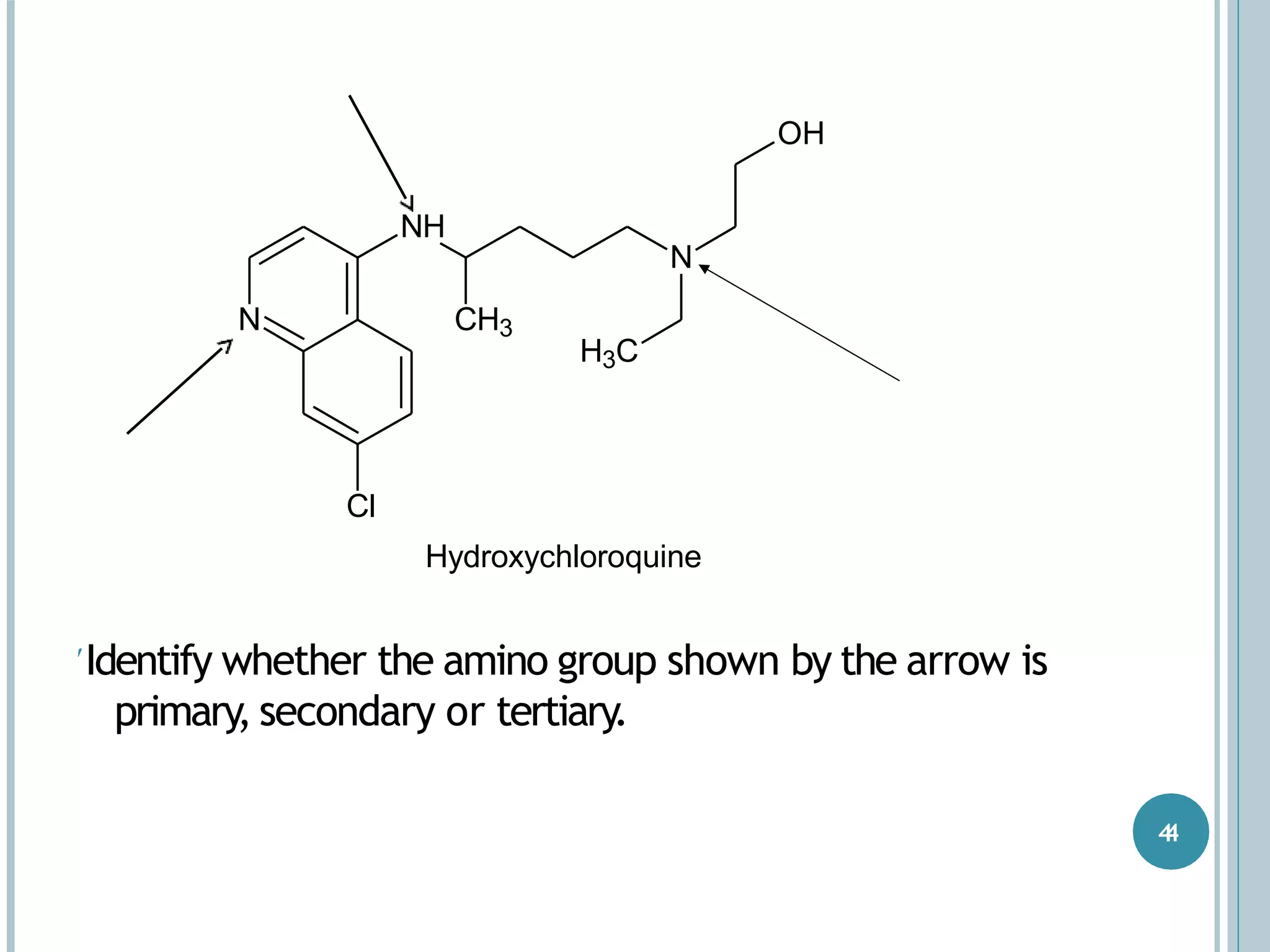
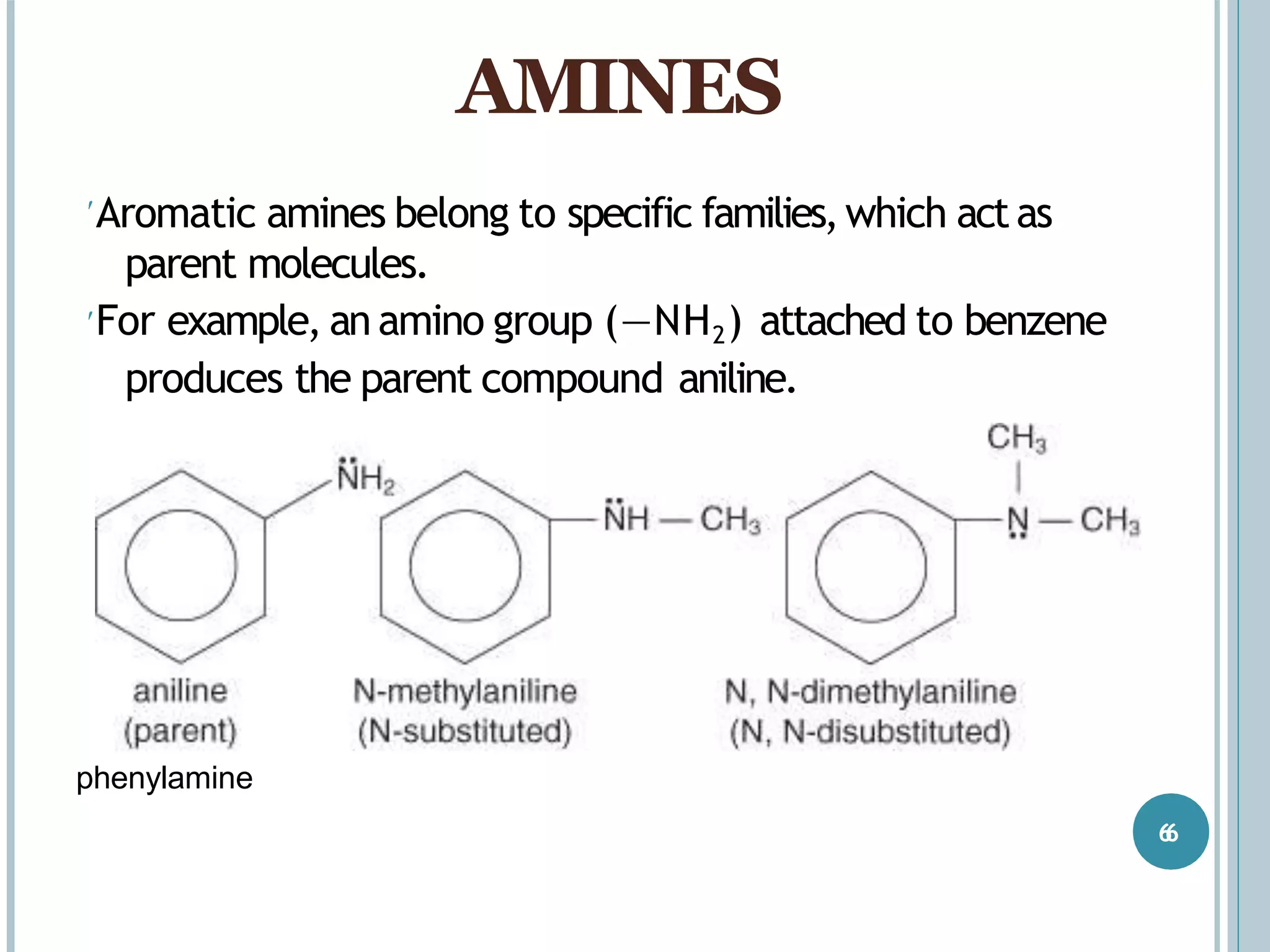
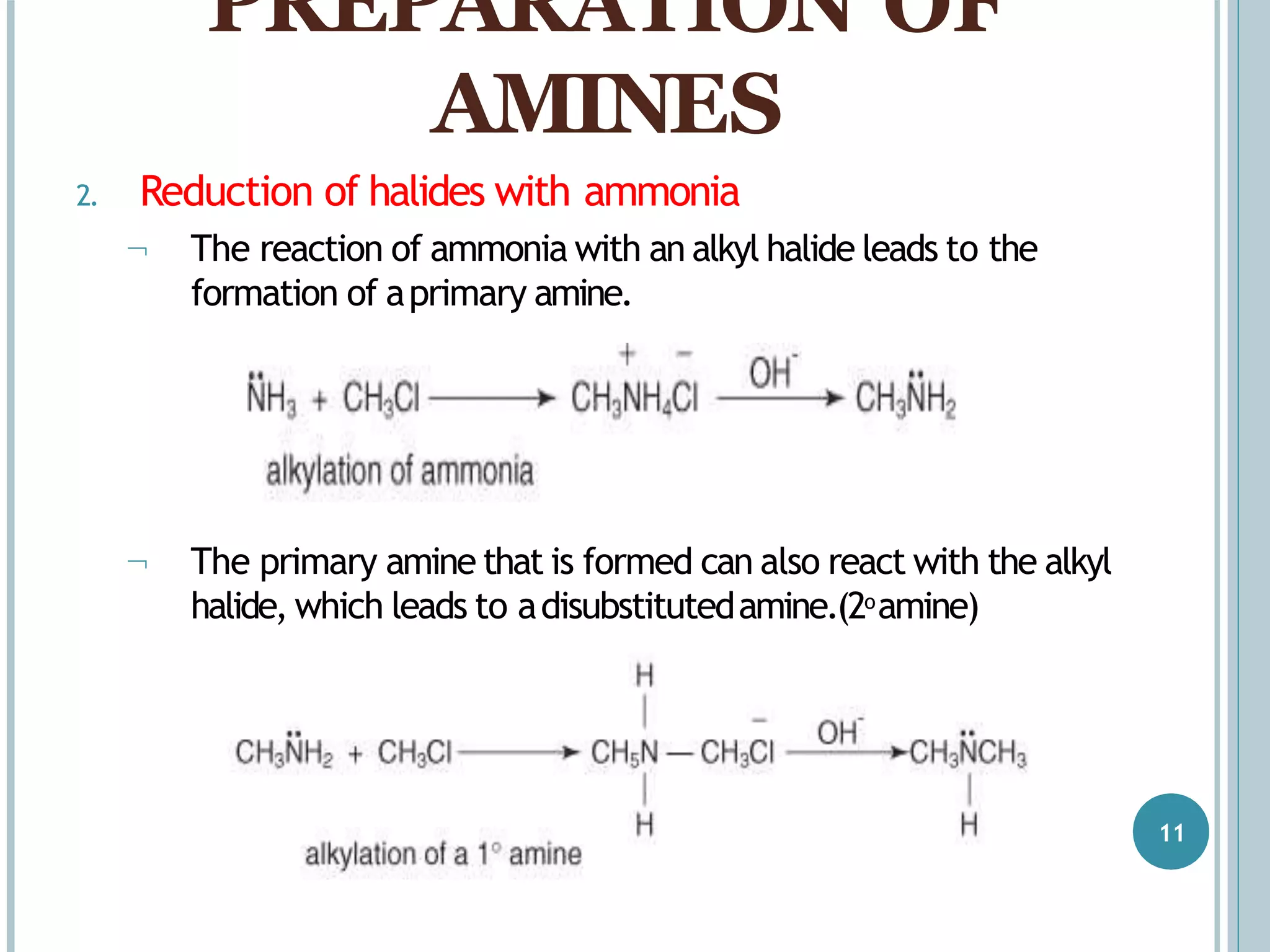
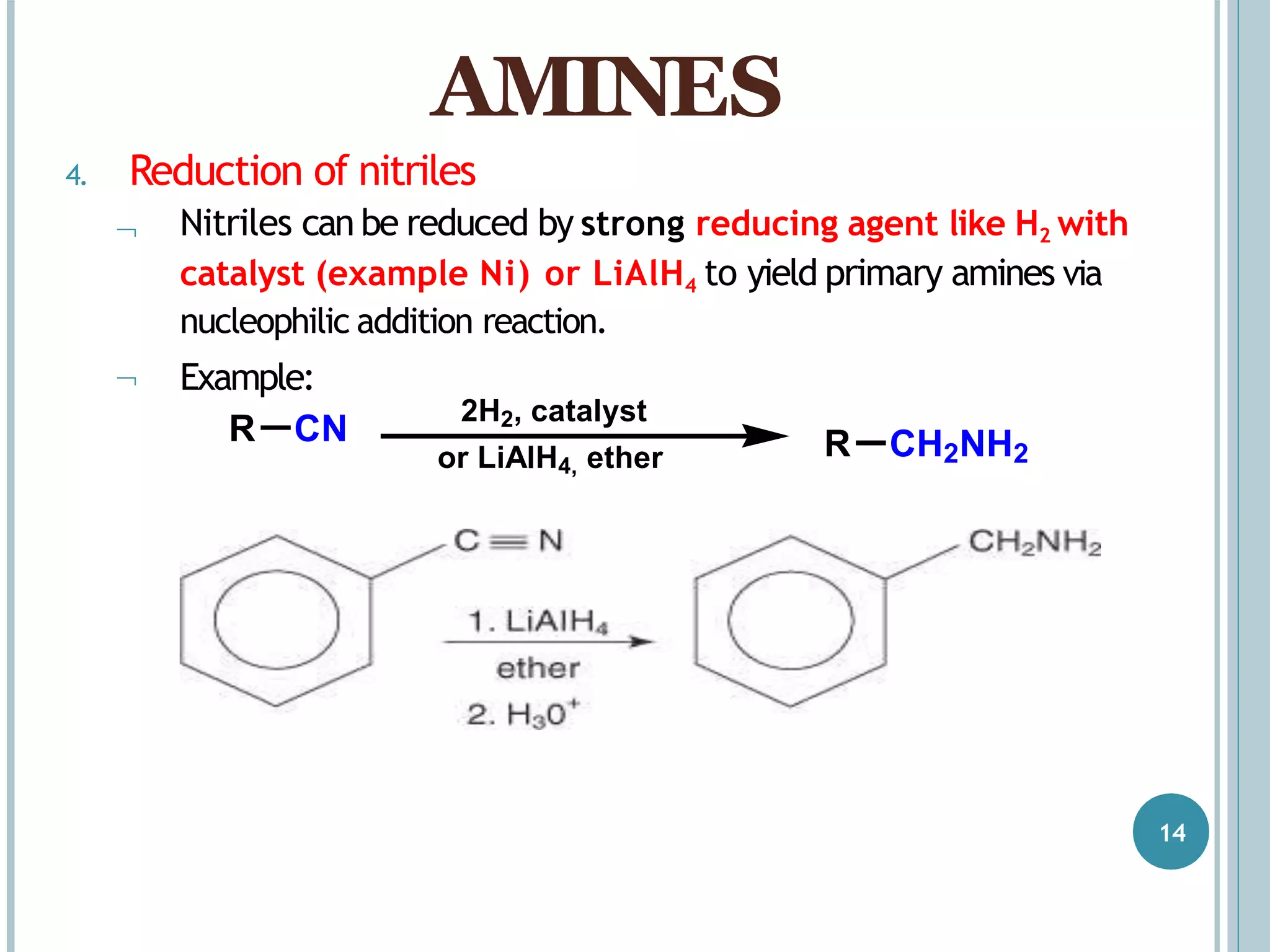
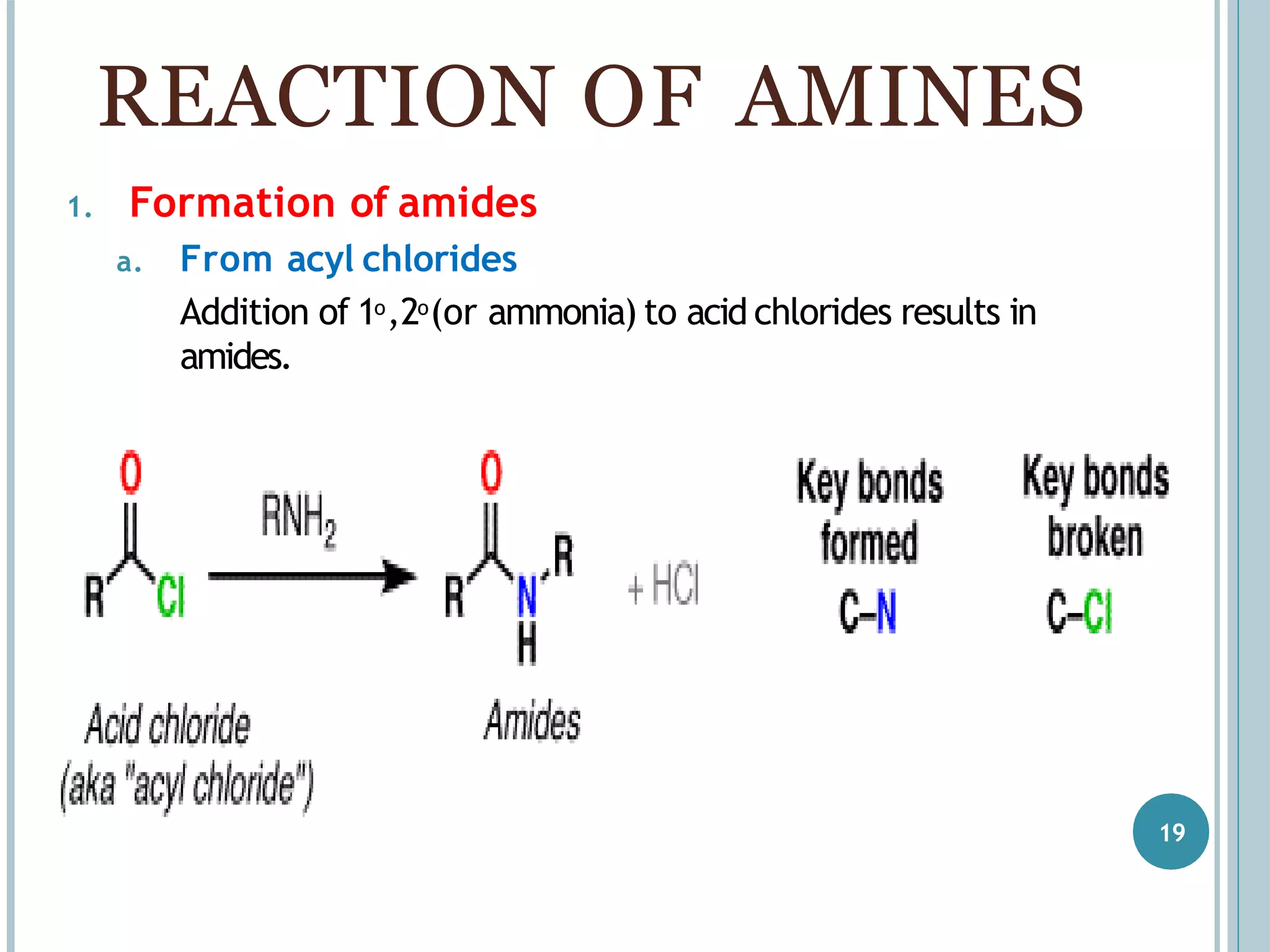
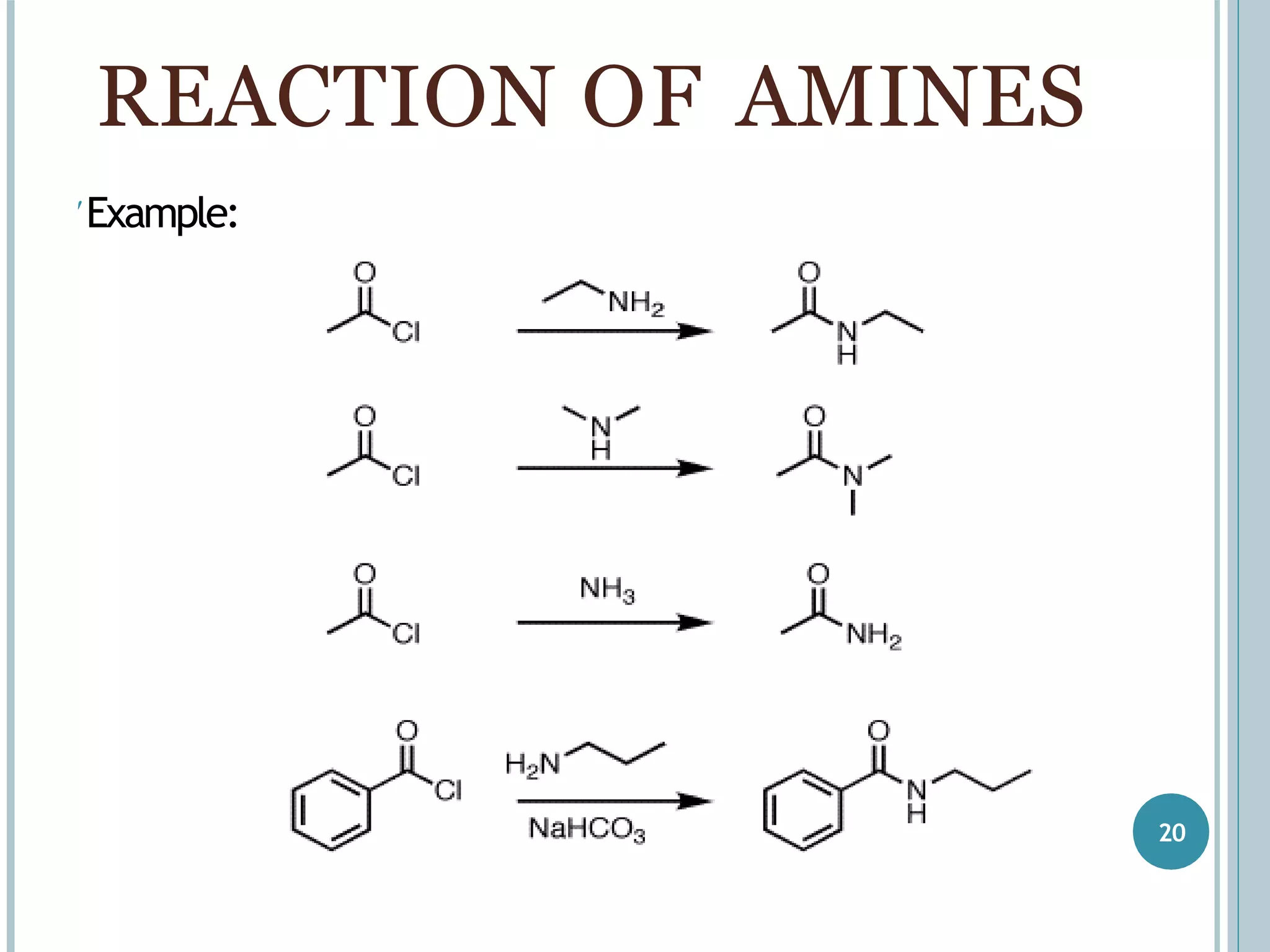
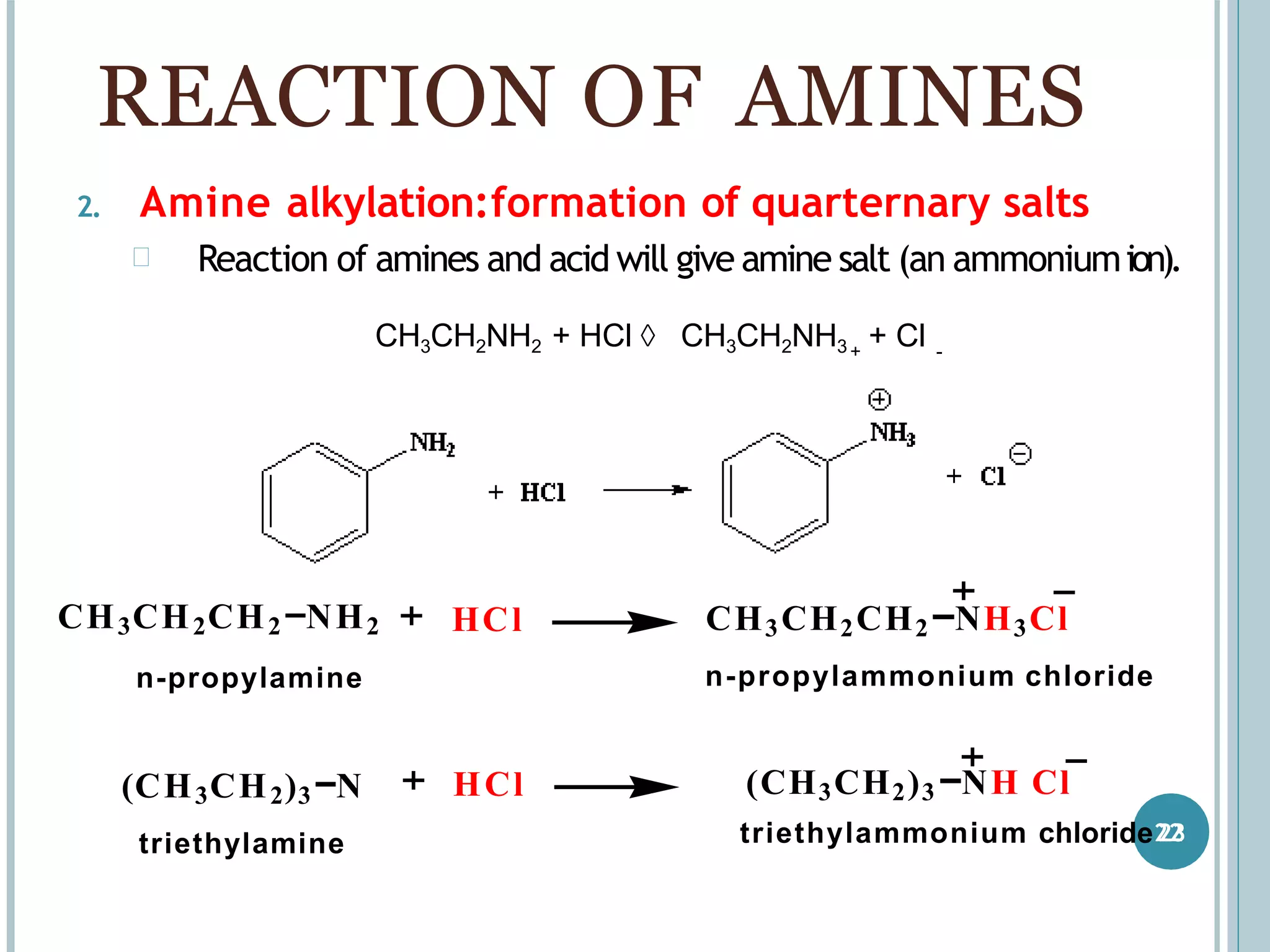
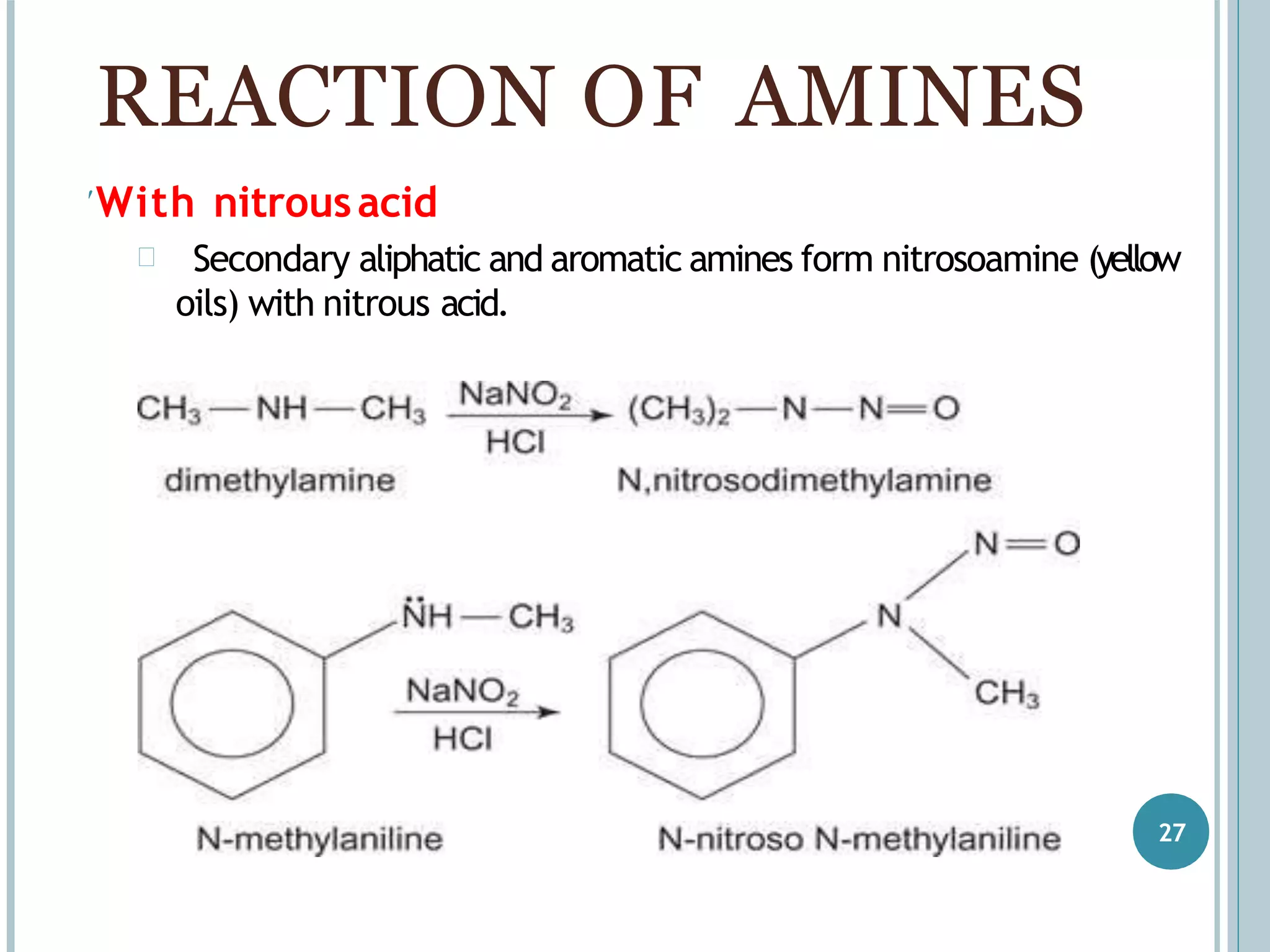
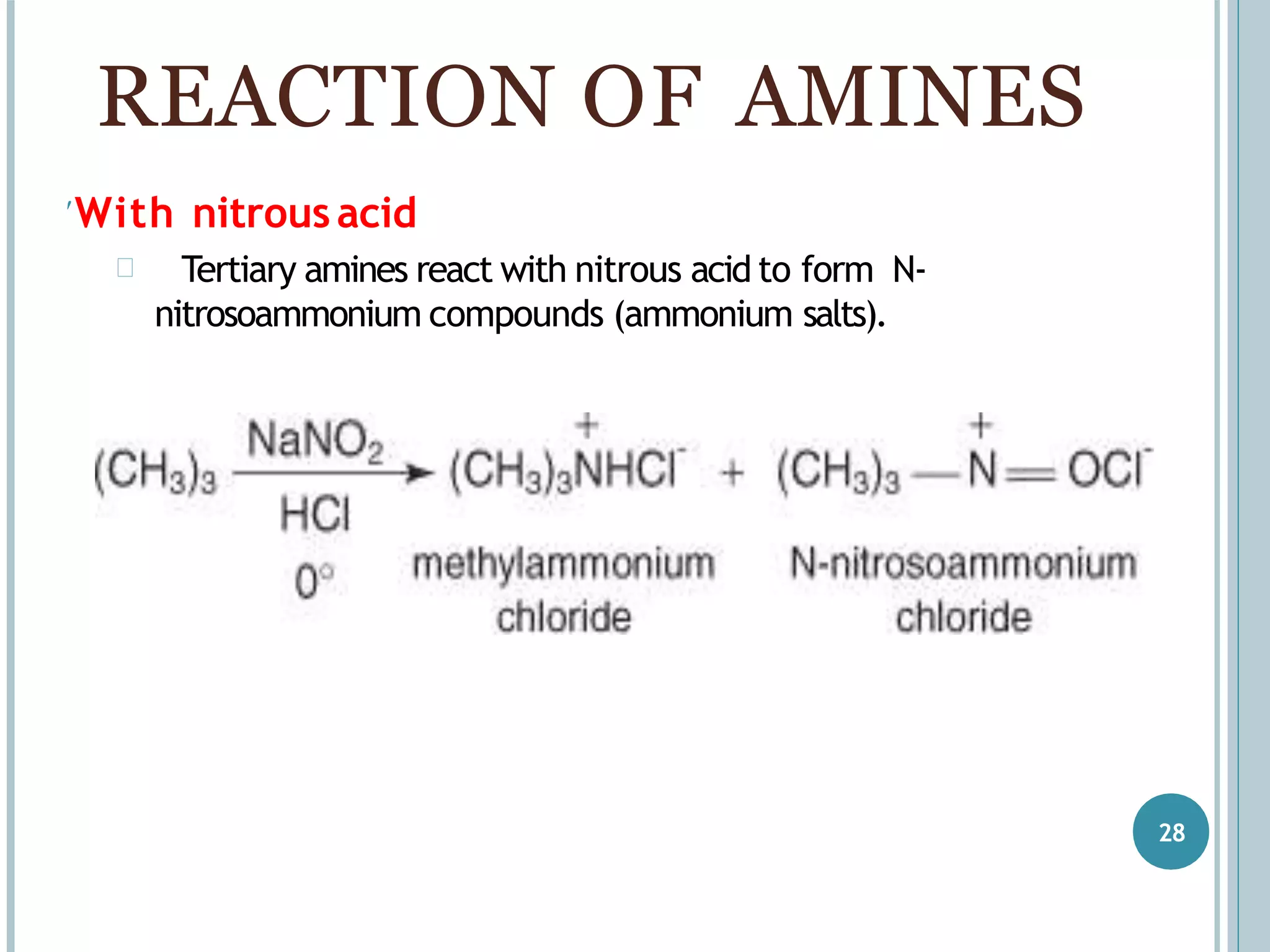
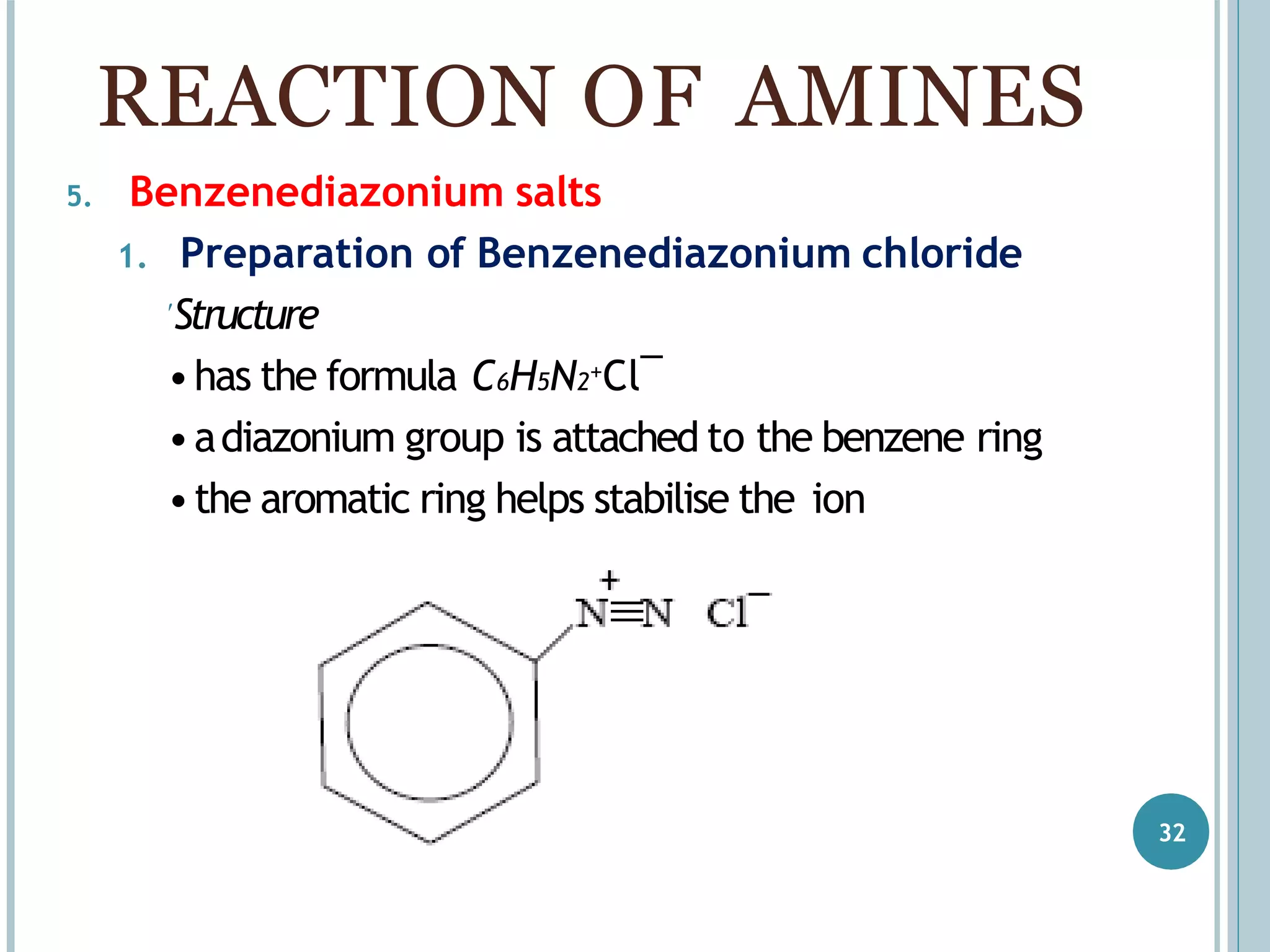
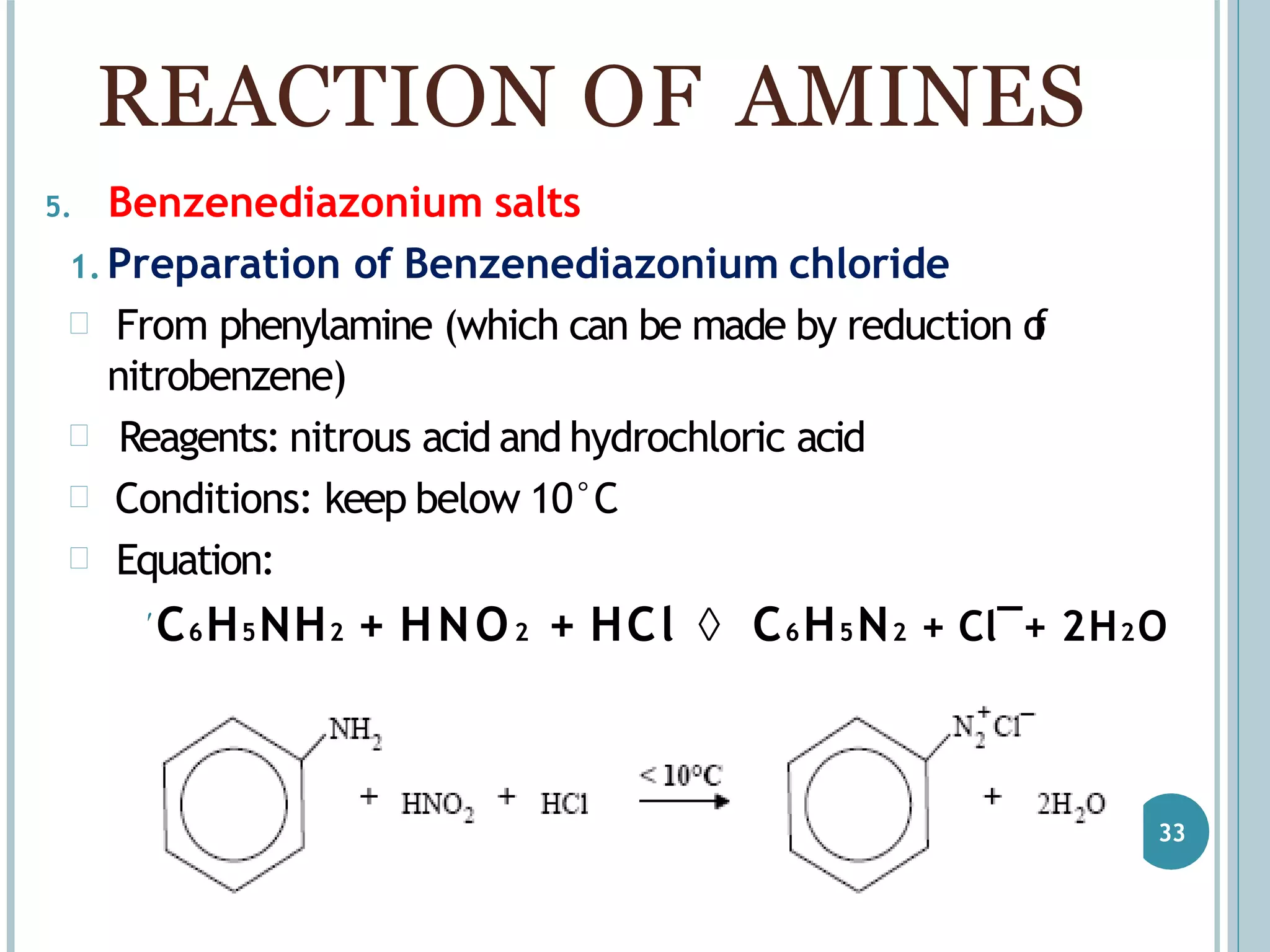
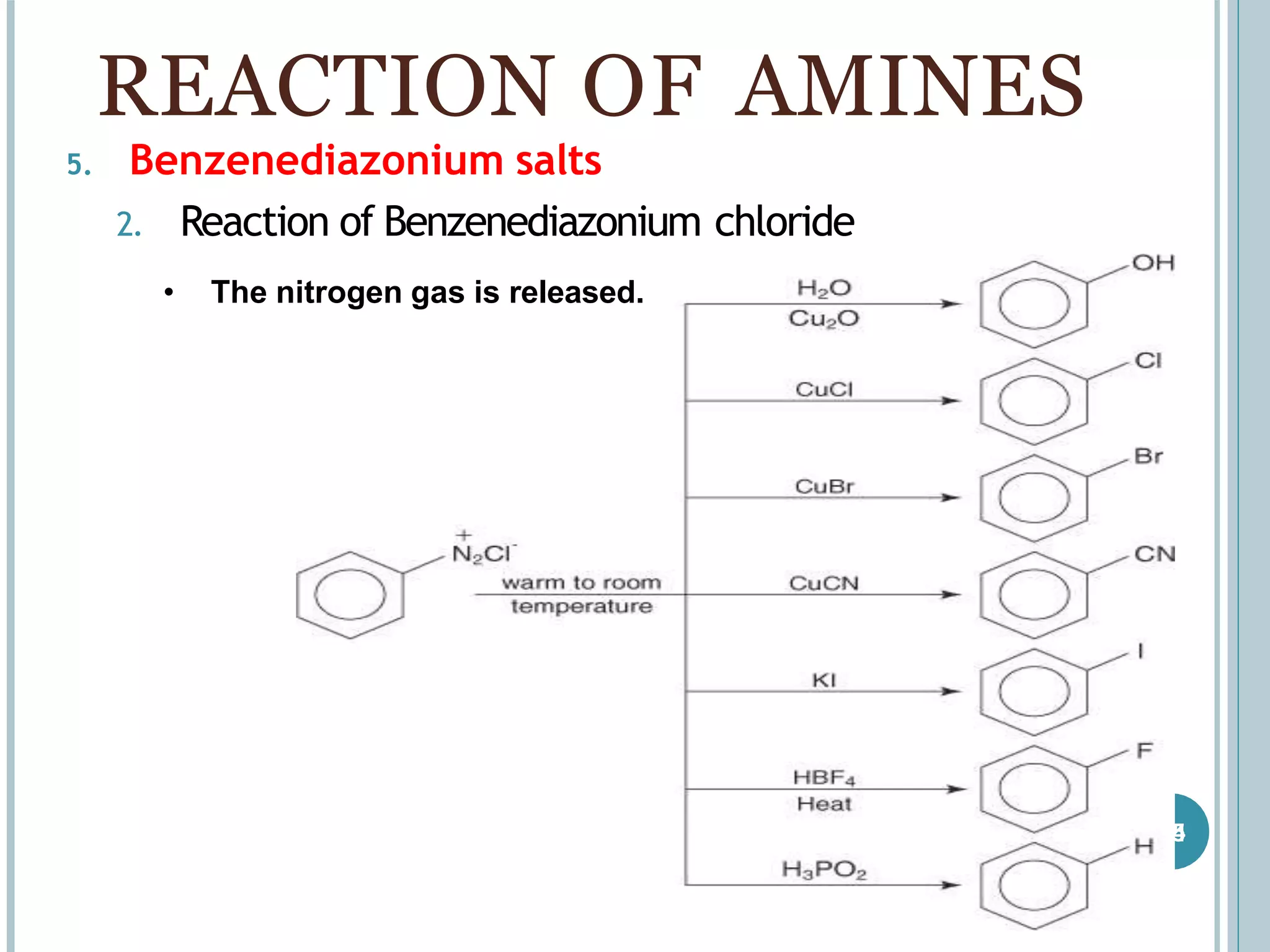
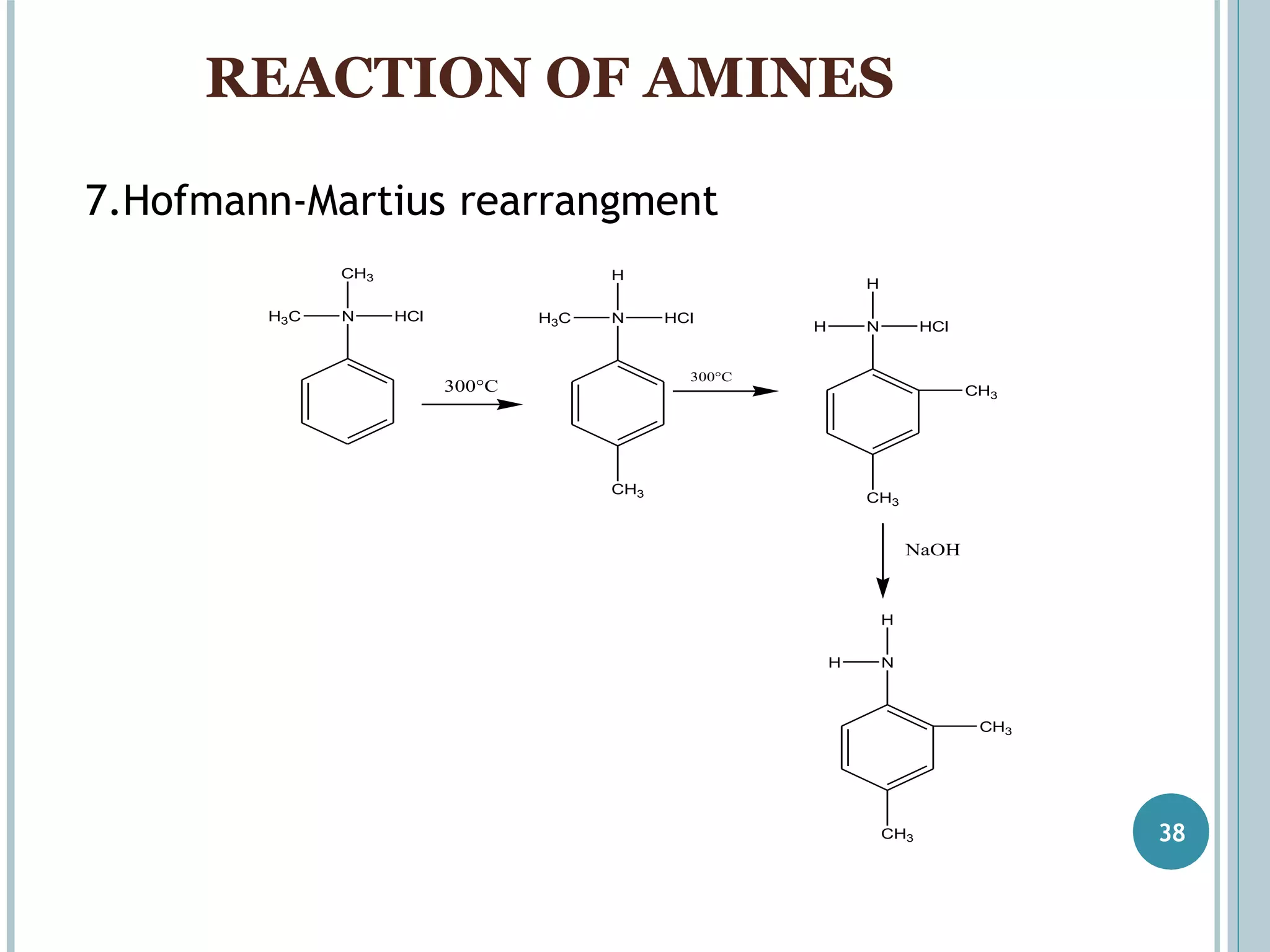
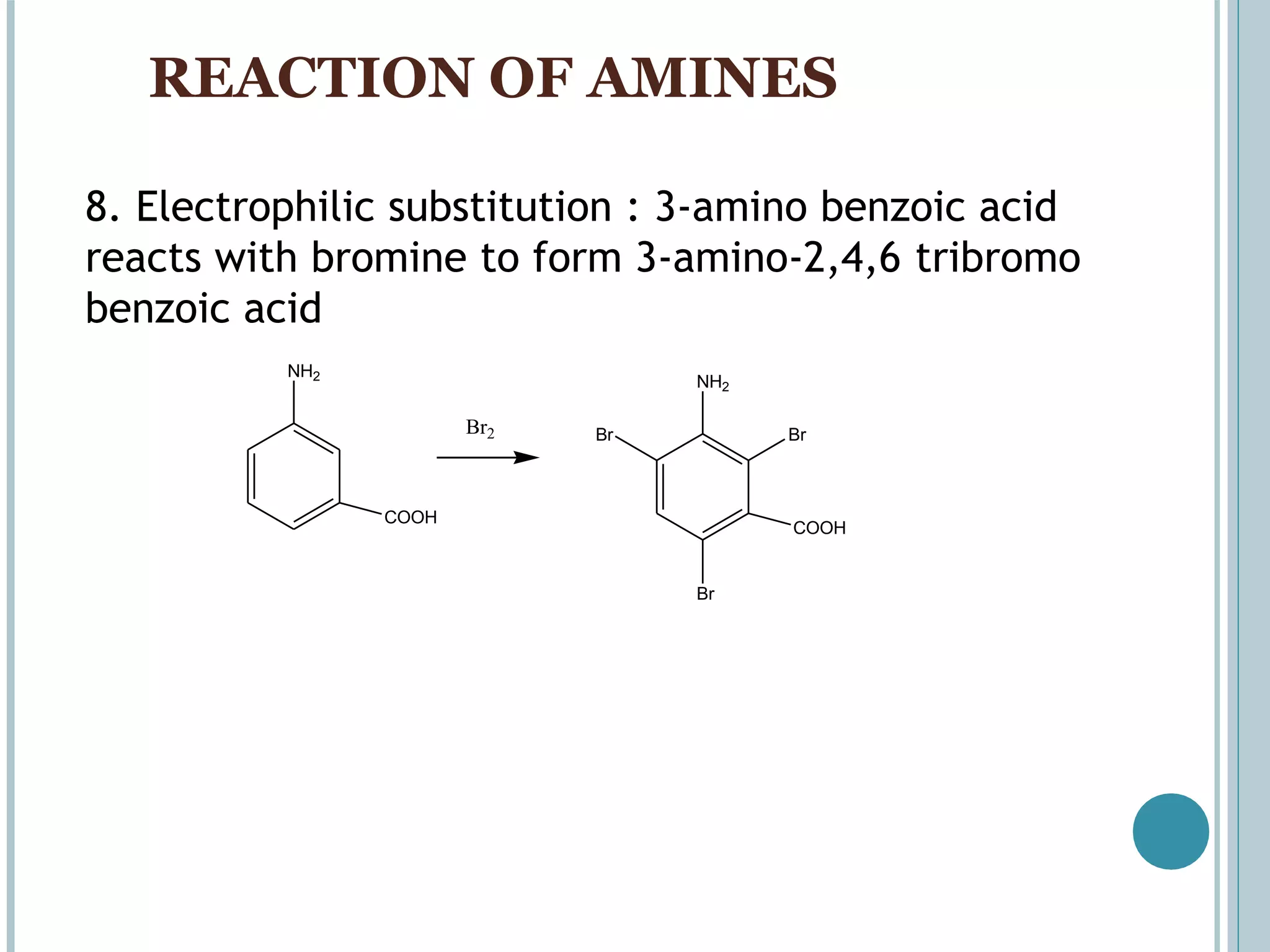
The document discusses aromatic amines, their classification, preparation methods, and chemical properties. It explains how aromatic amines are derived from aromatic hydrocarbons, primarily through the reduction of nitro compounds, and highlights their basicity influenced by electron-donating or withdrawing groups. Various reactions of amines, including their interactions with nitrous acid and the formation of diazonium salts, are also summarized.