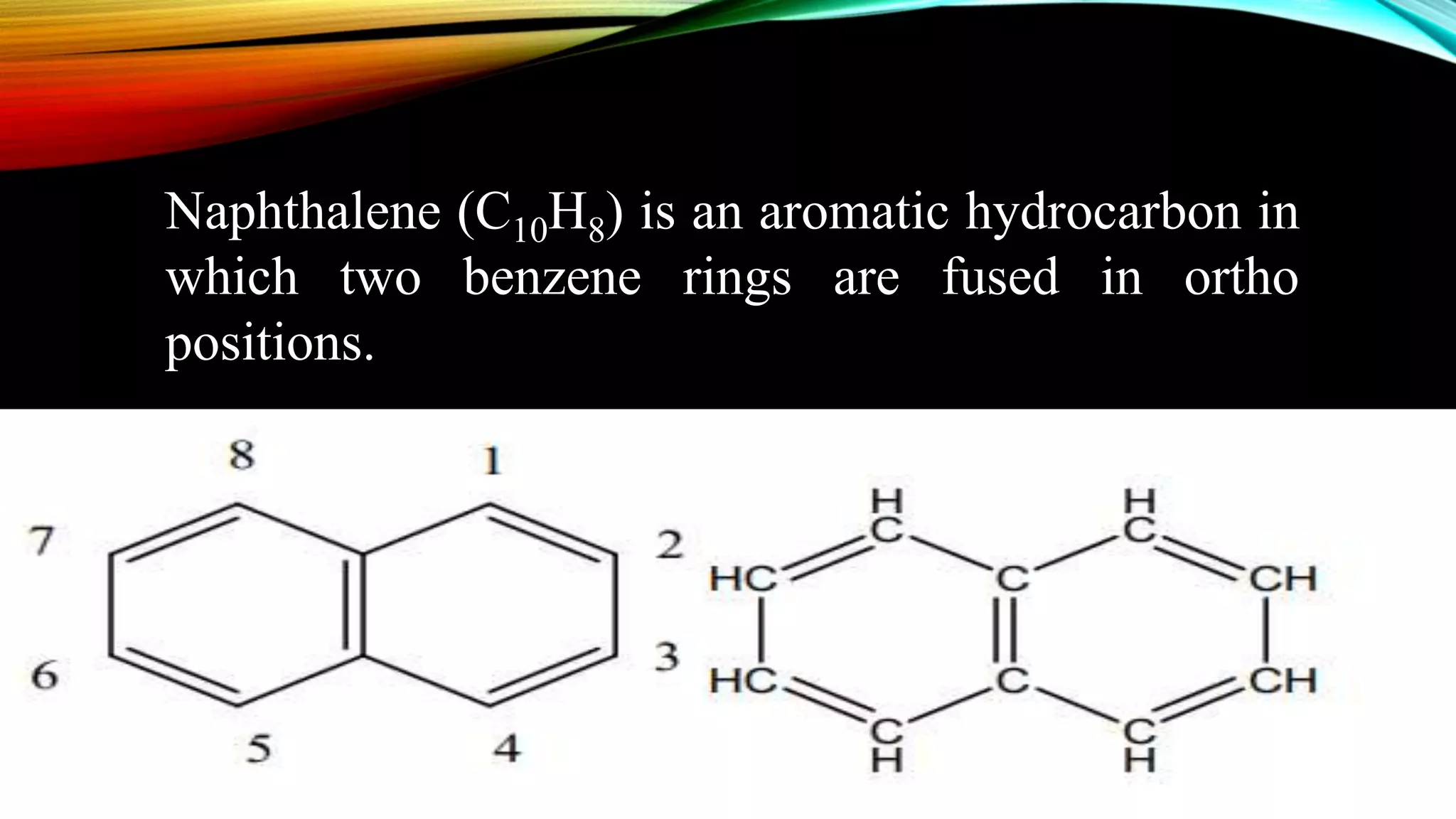
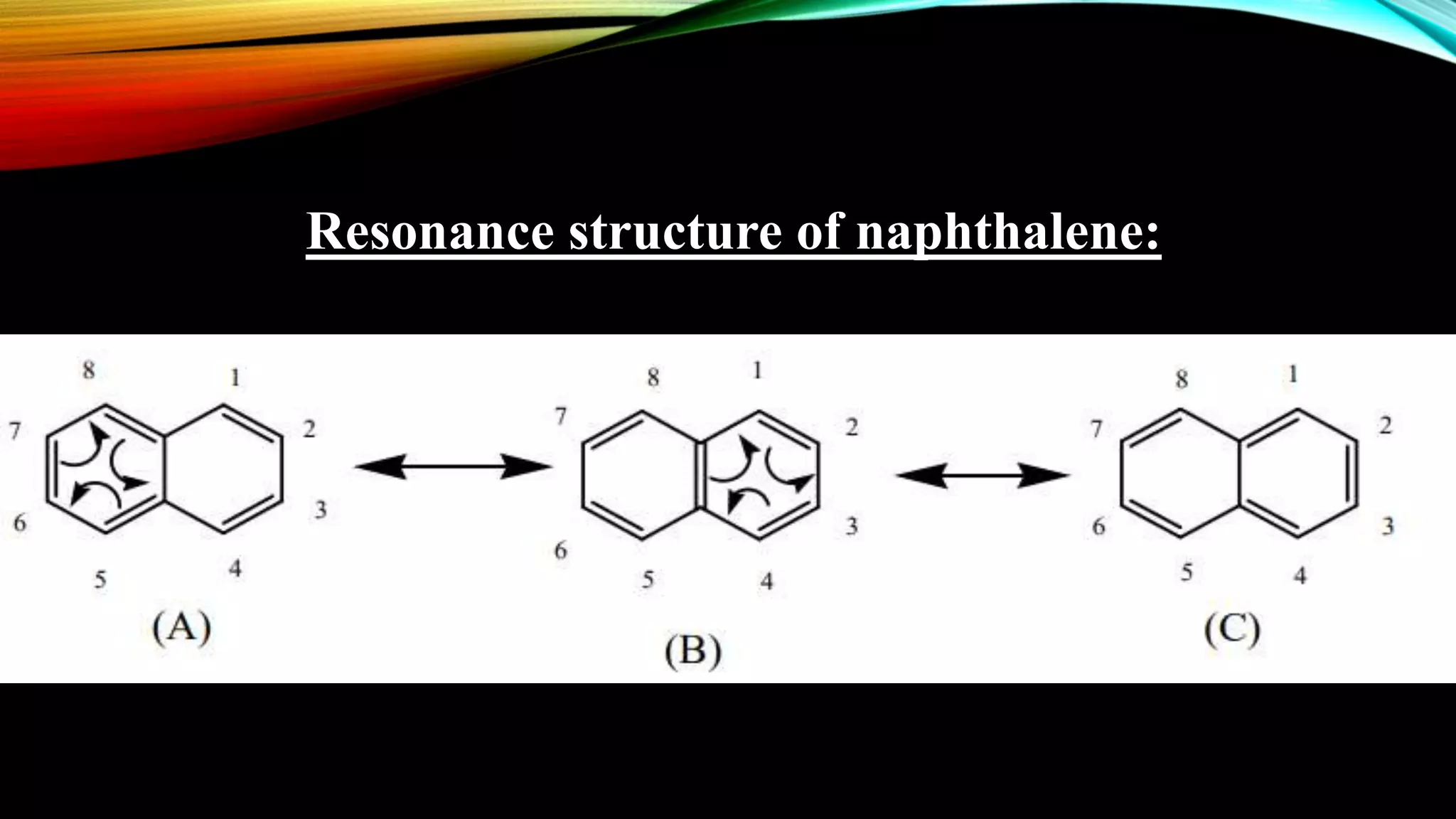
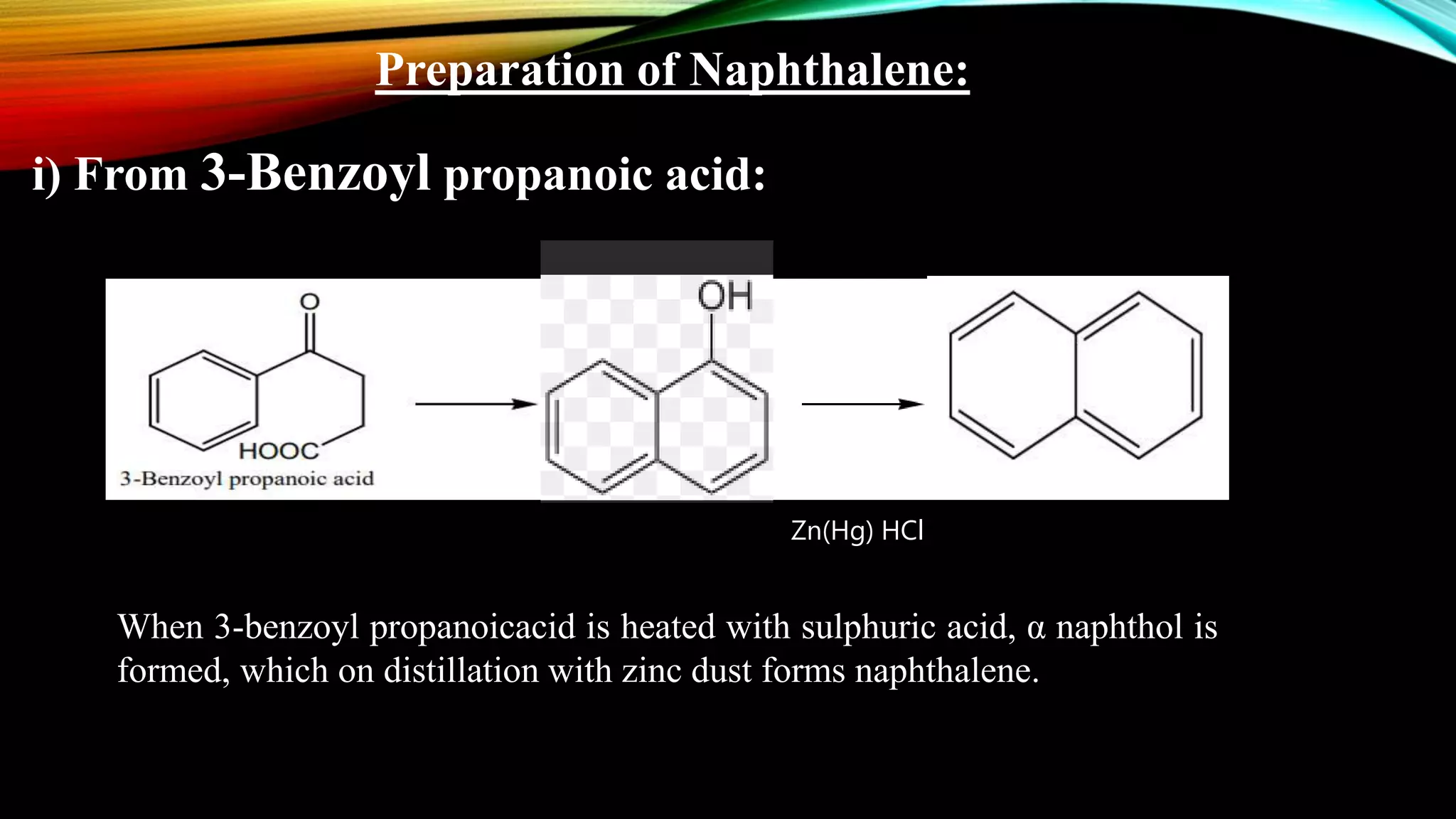
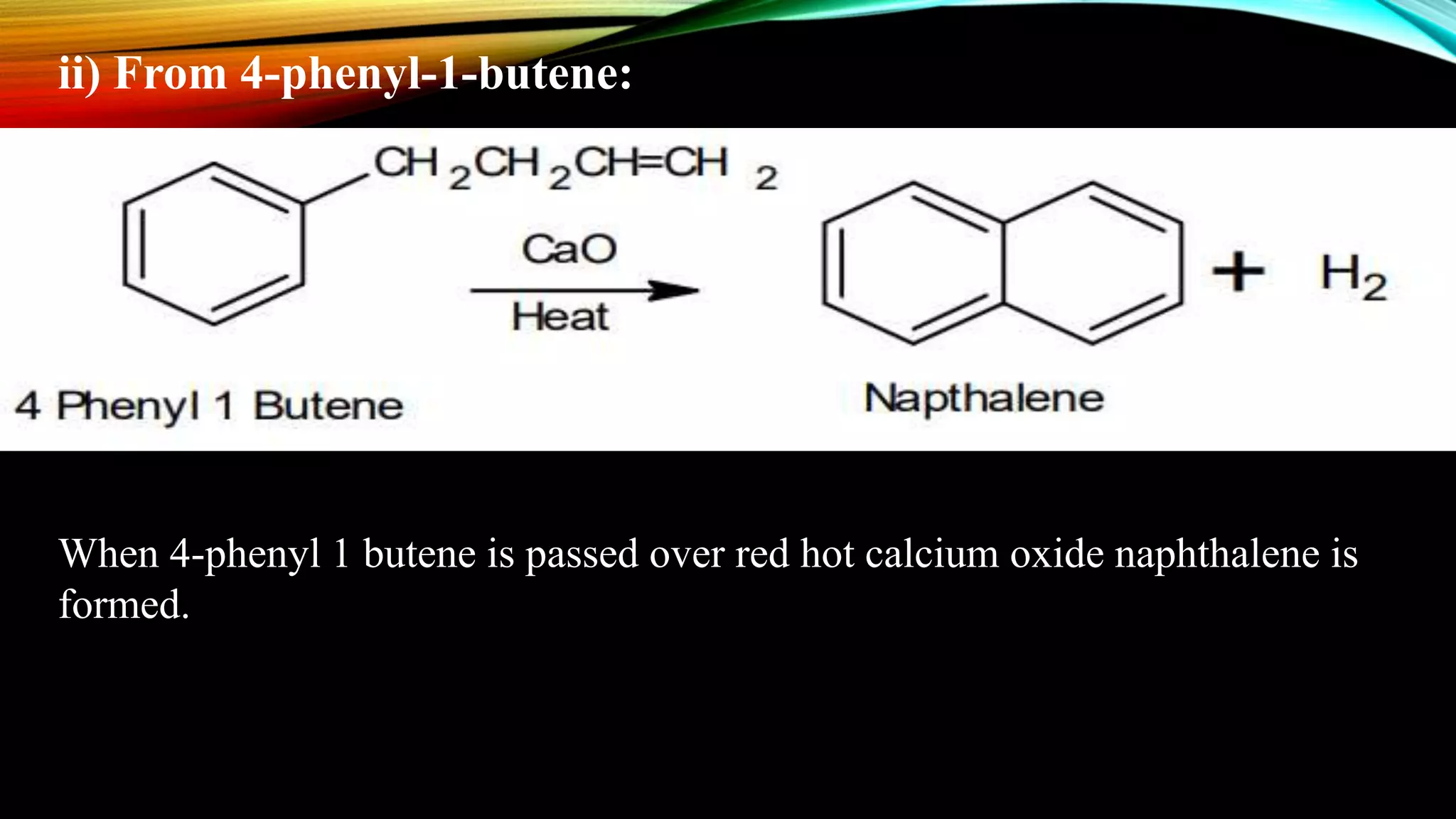
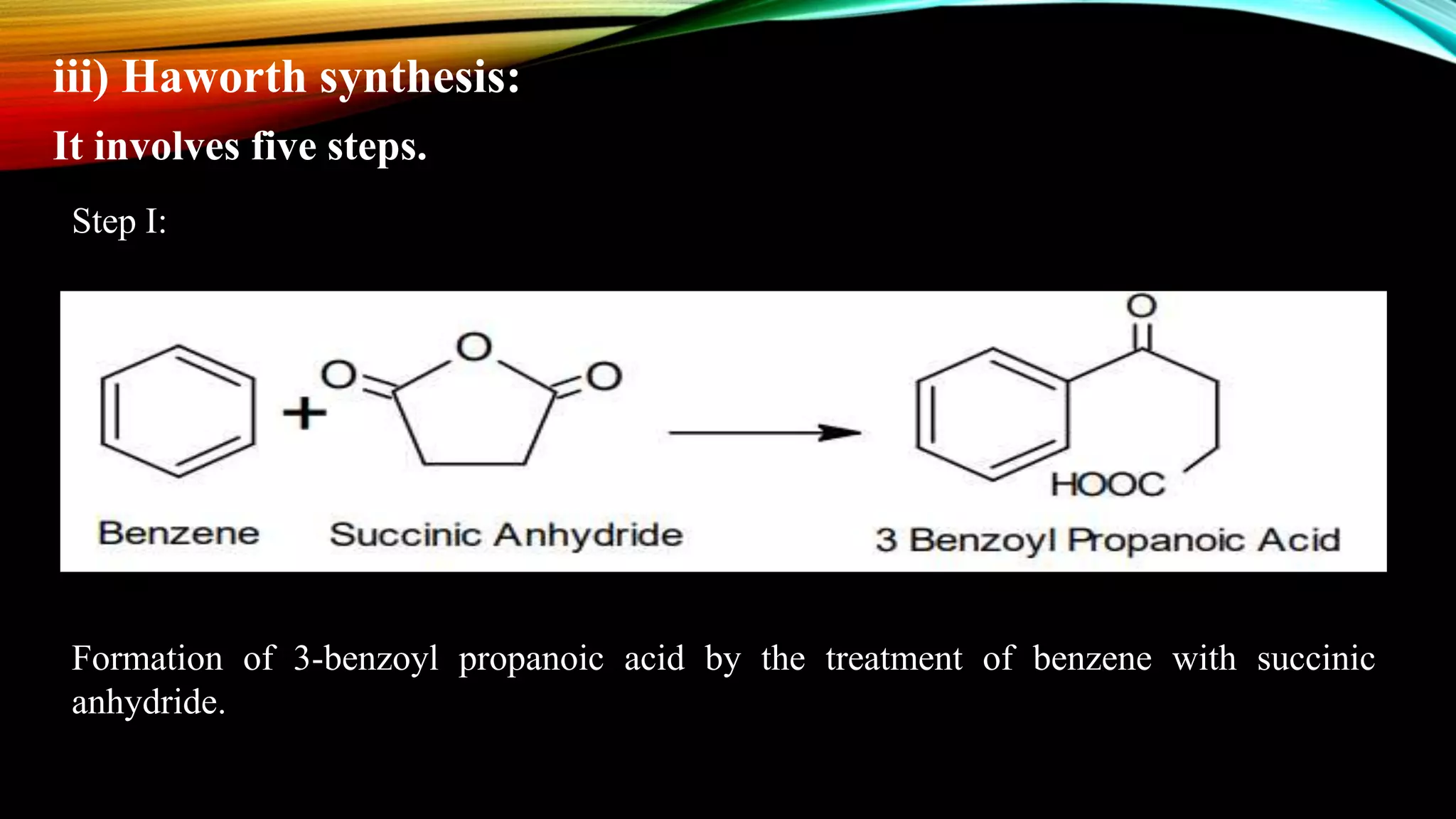
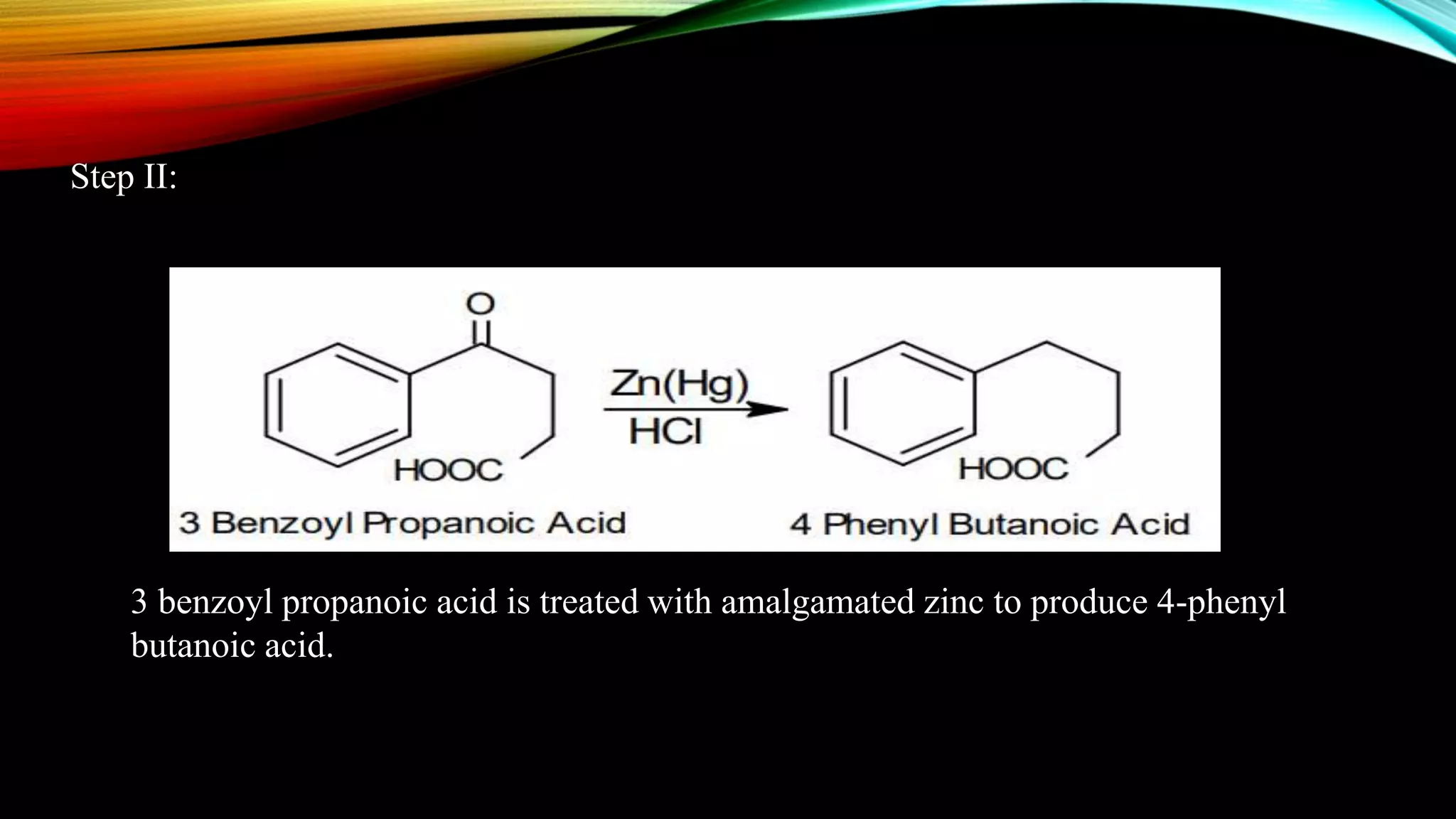
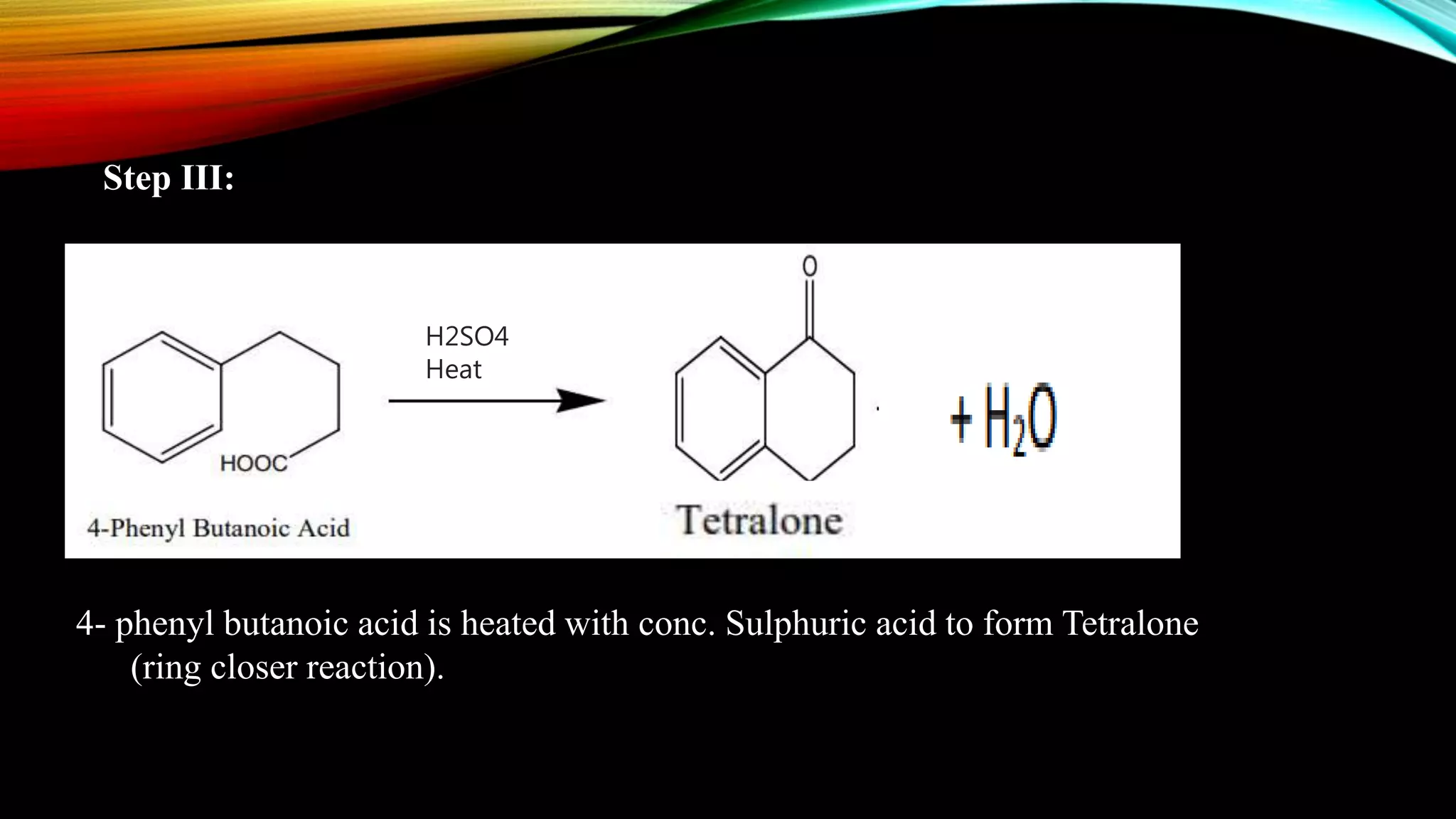
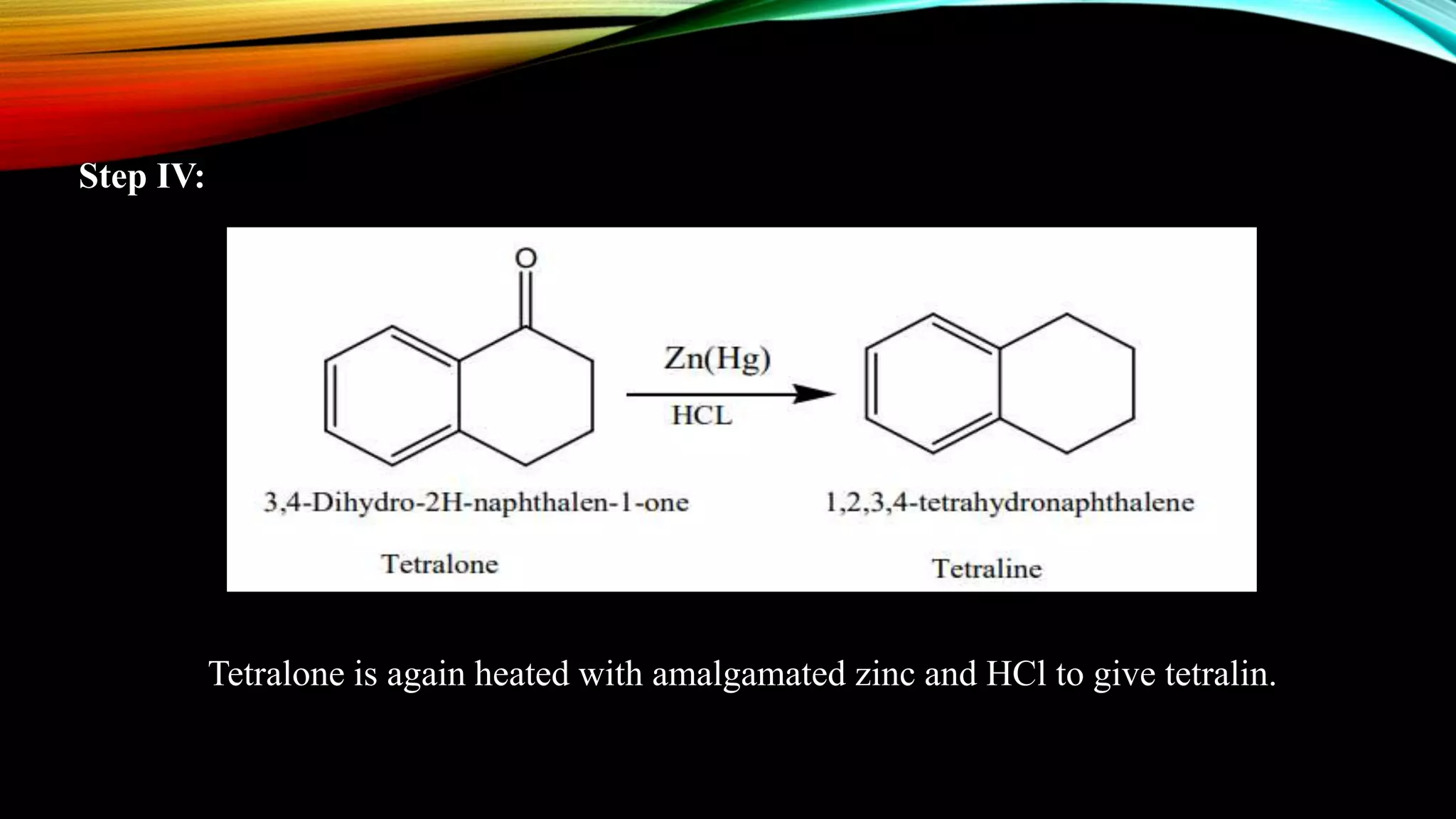
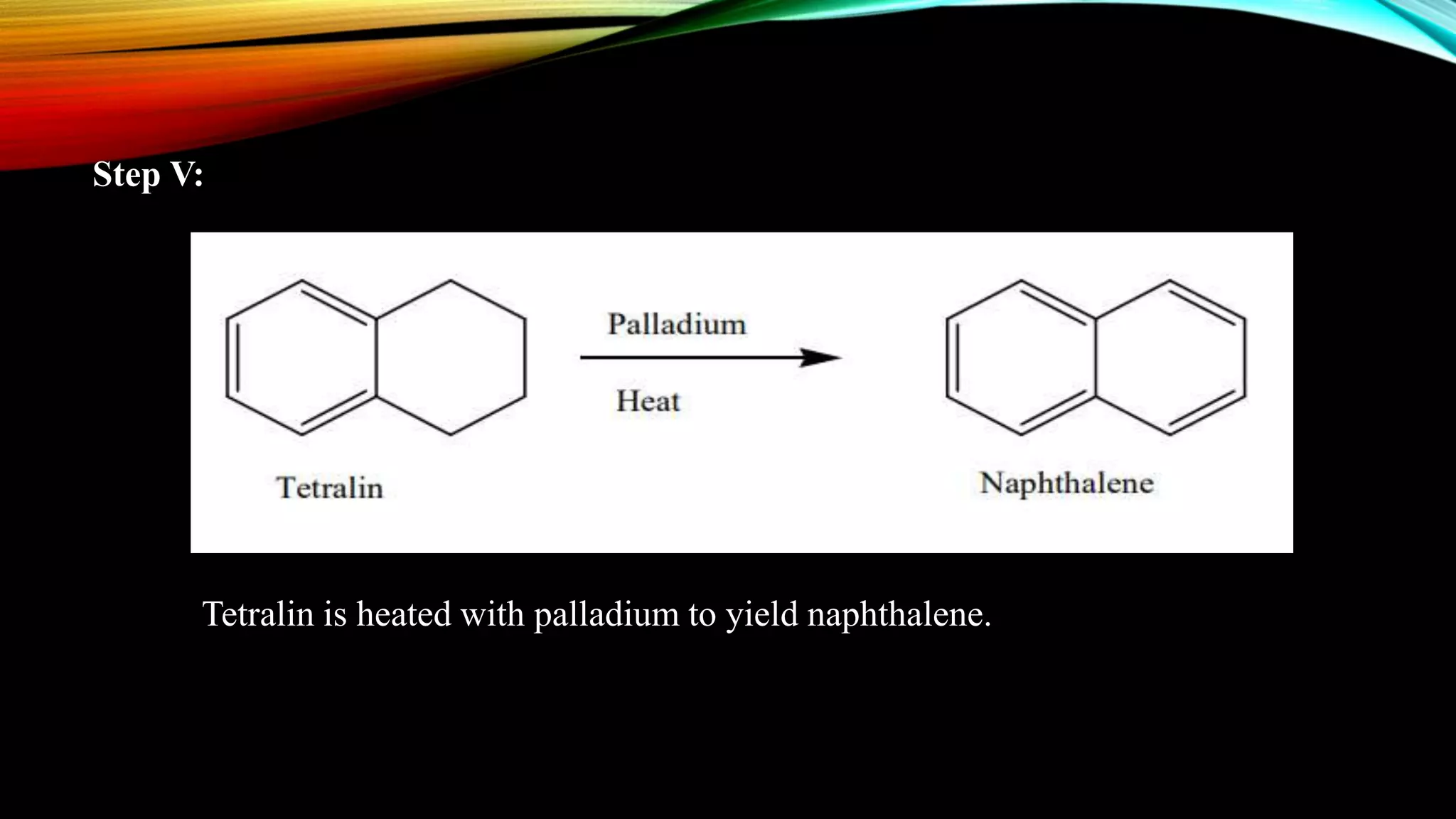
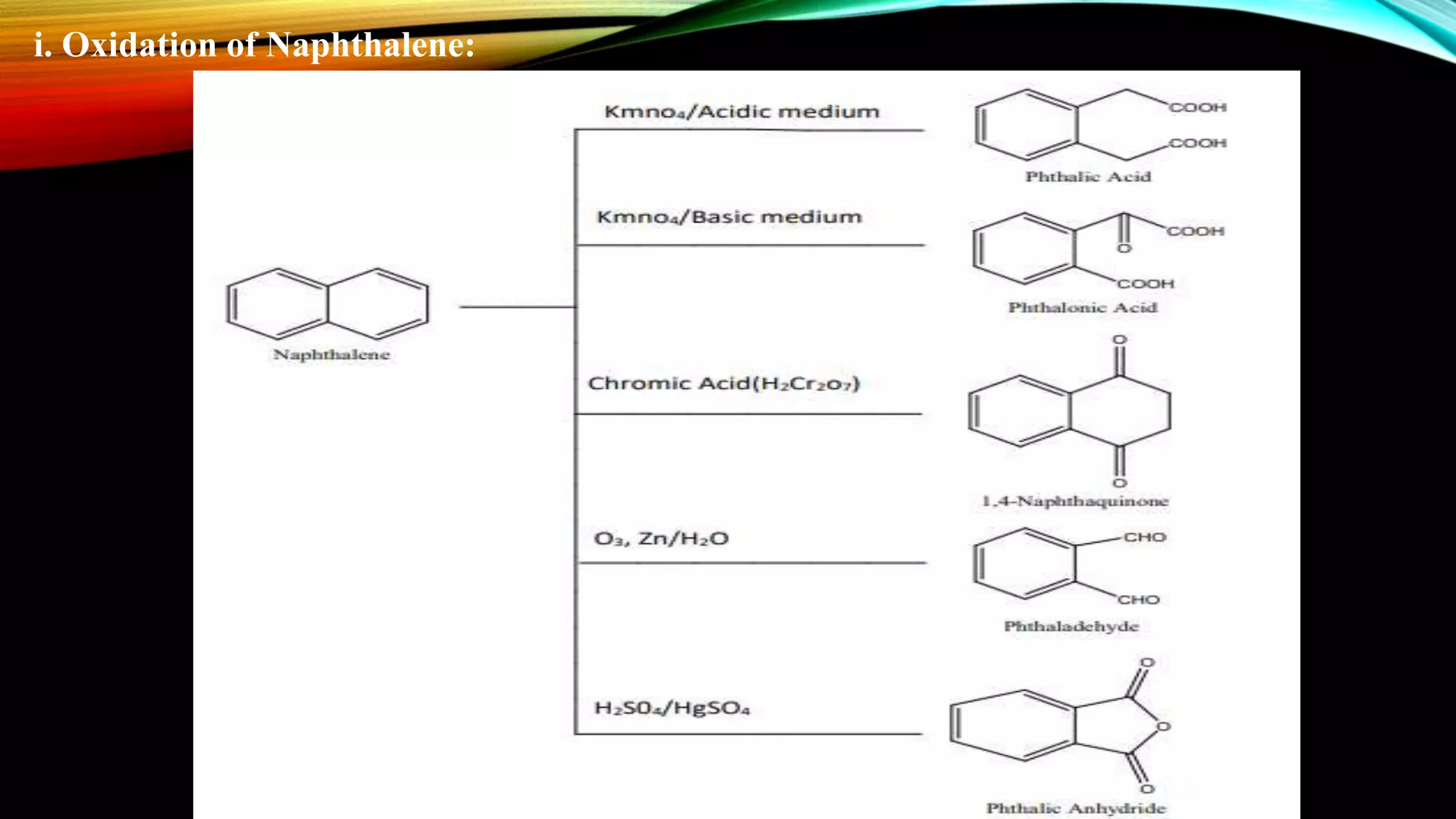
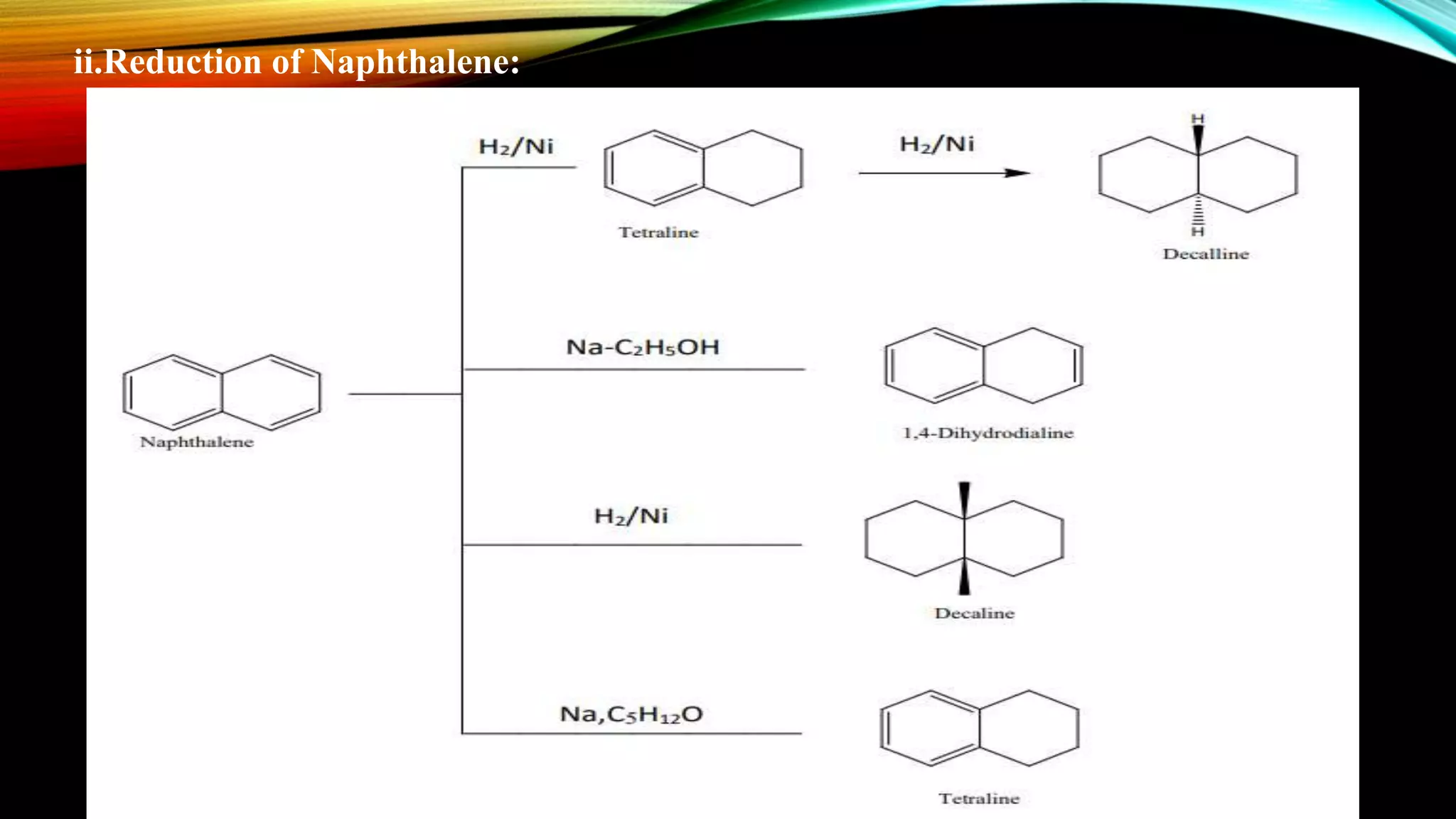
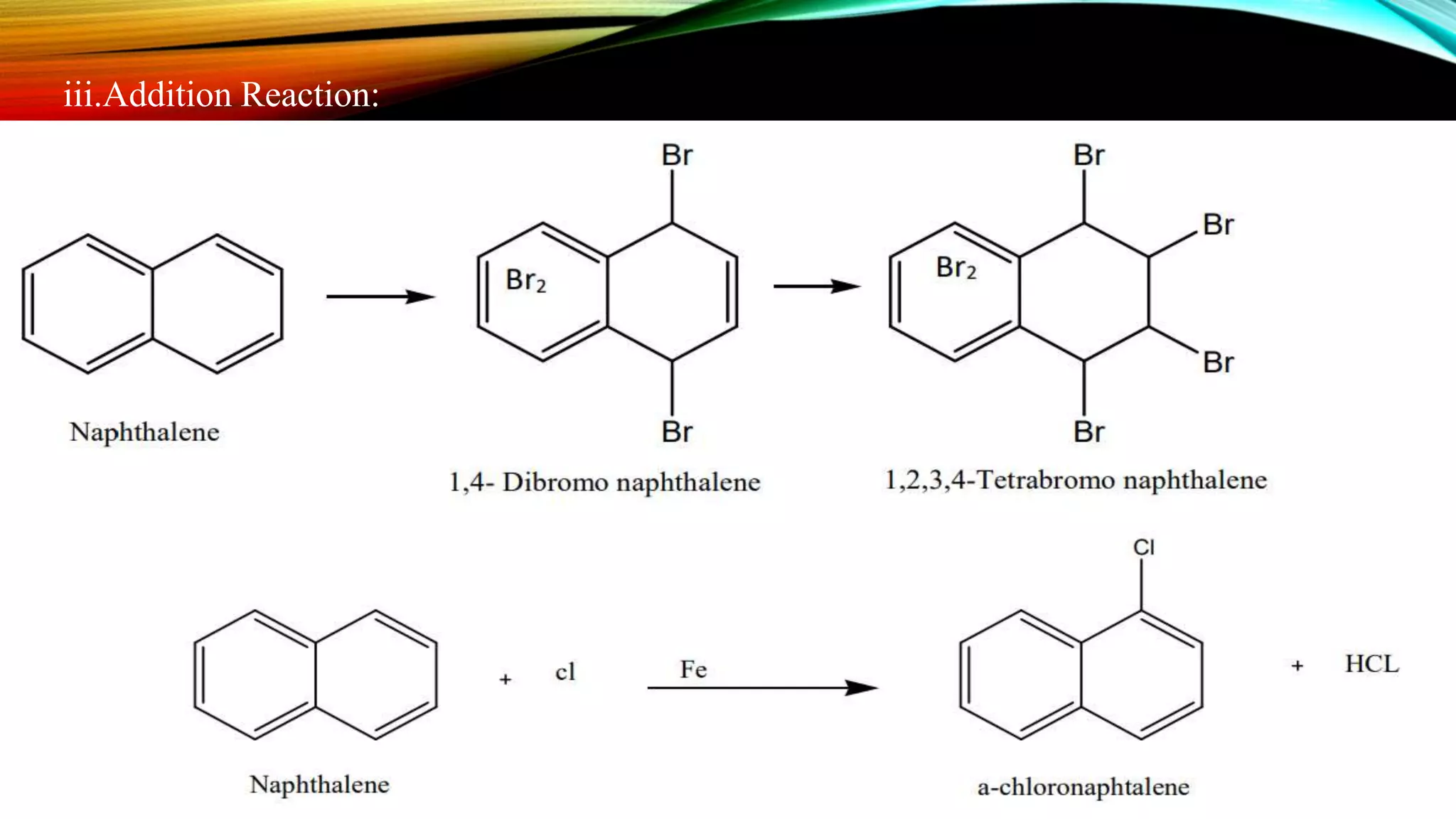
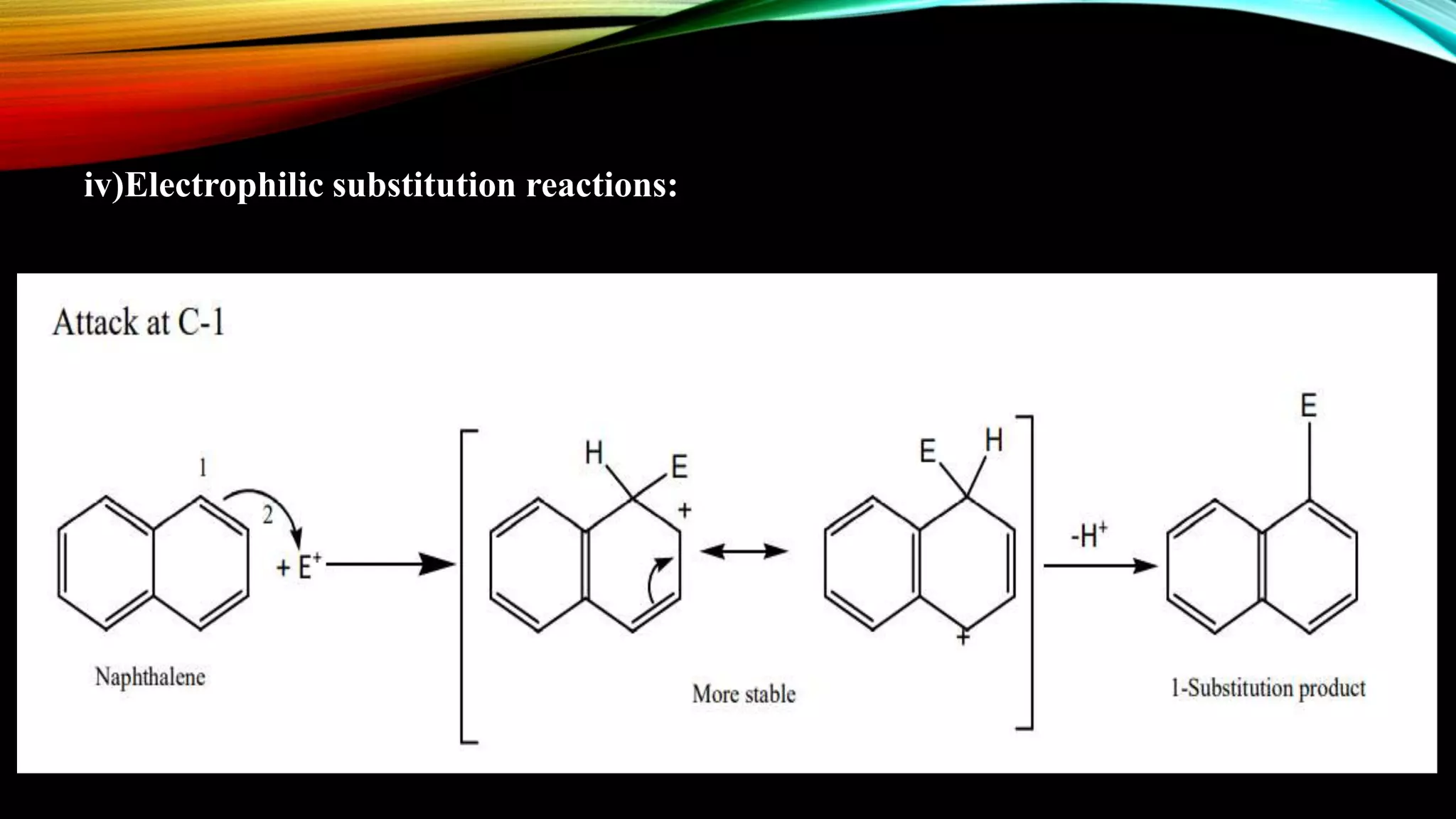
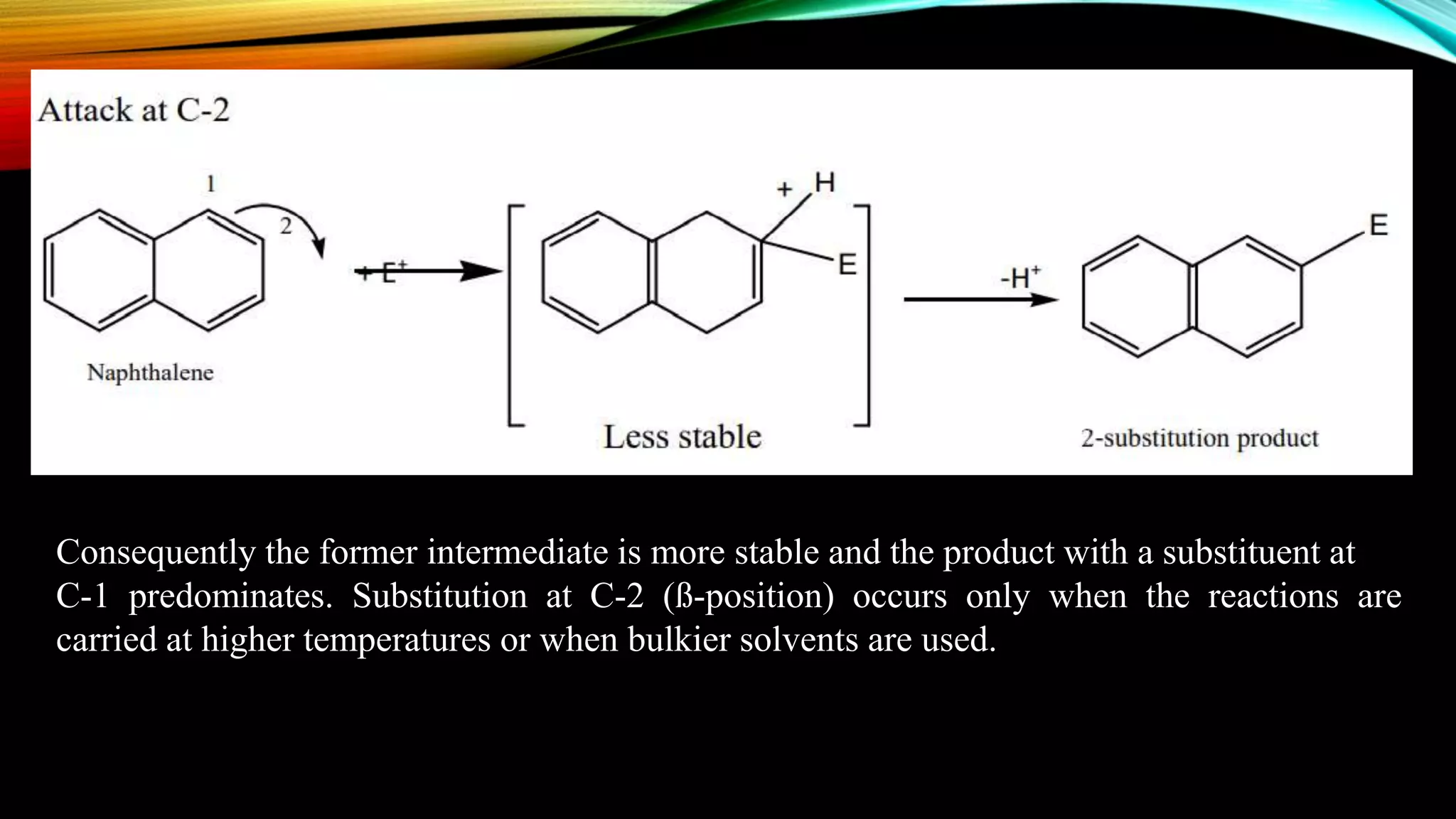
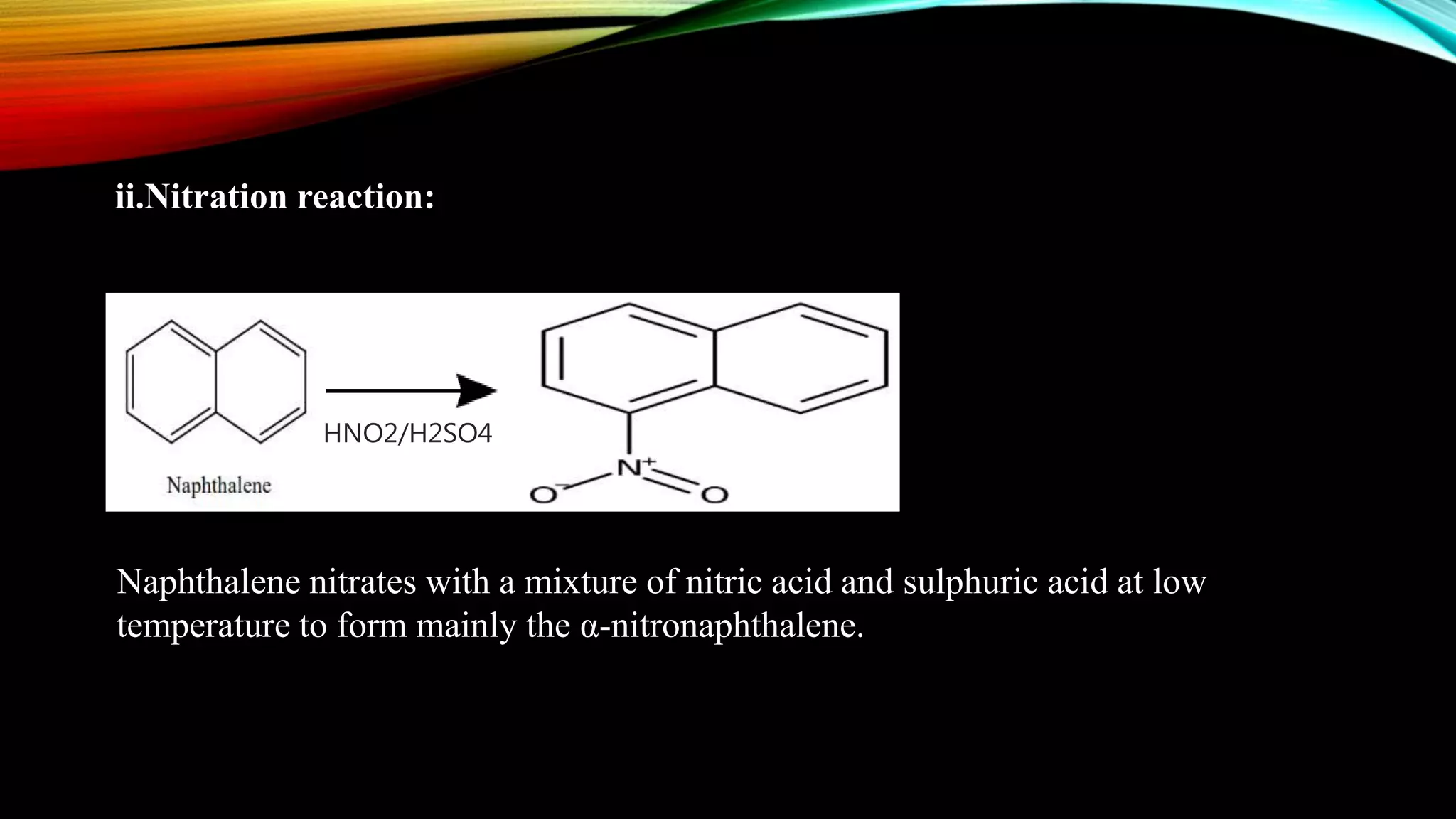
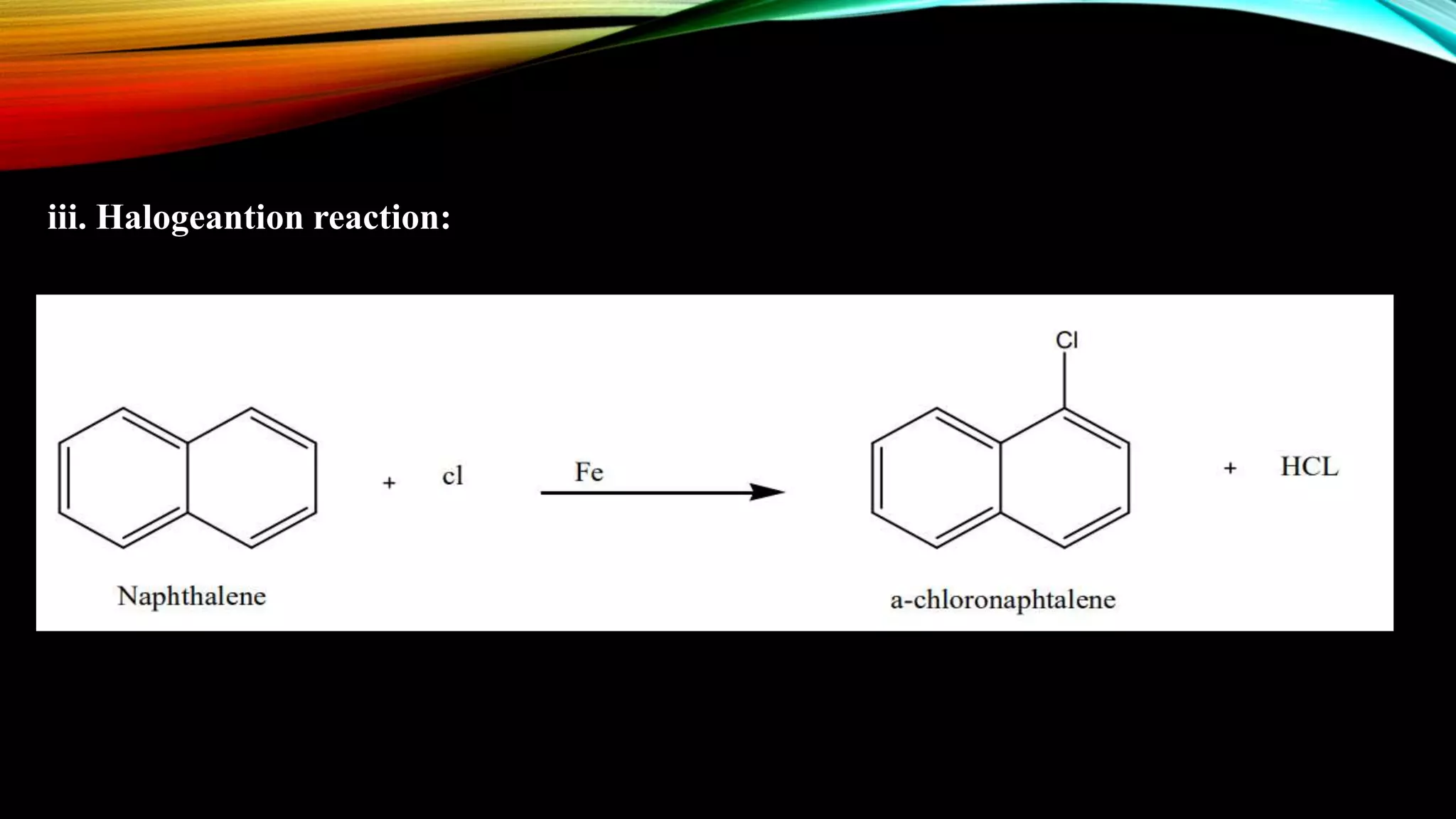
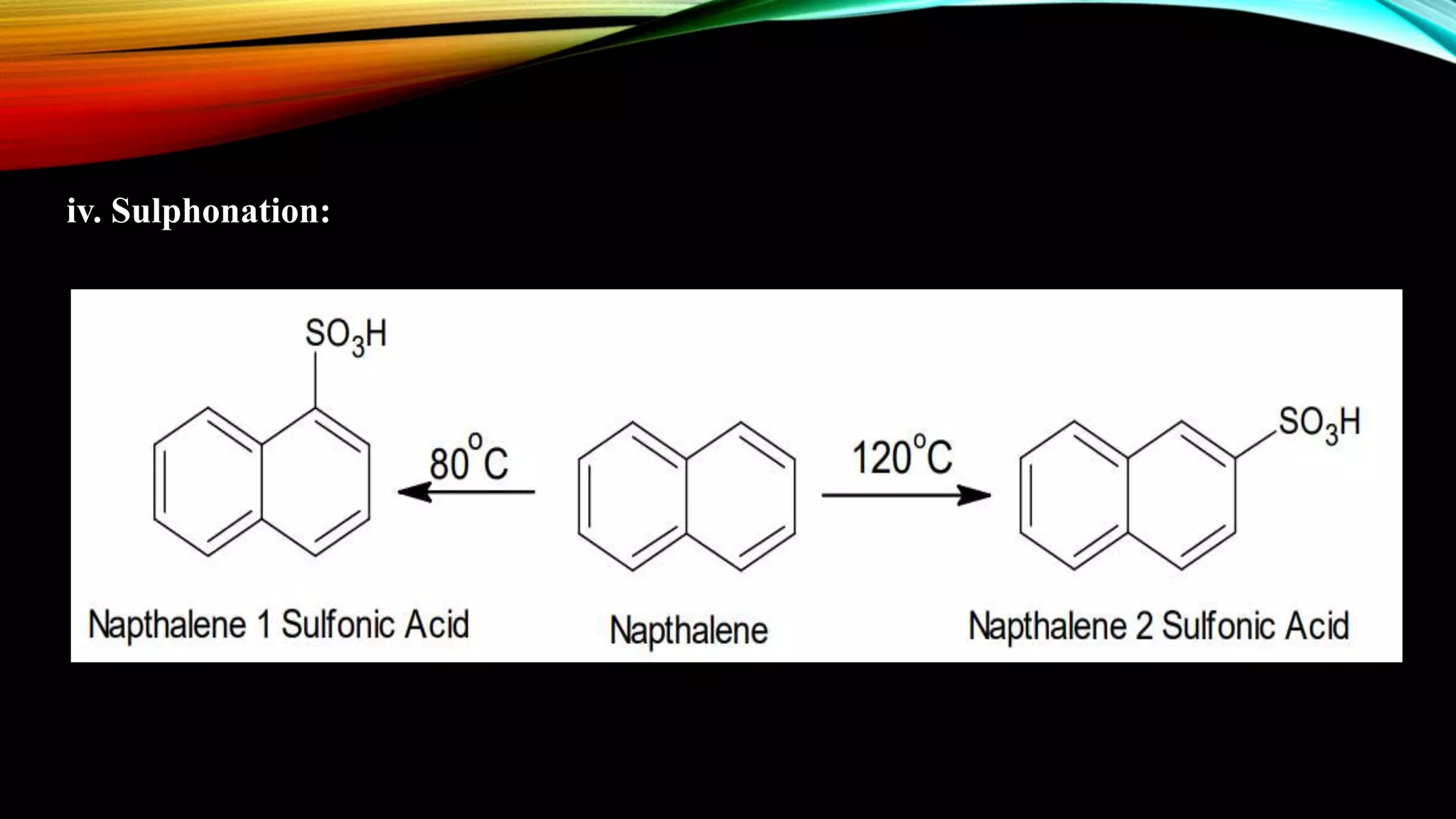
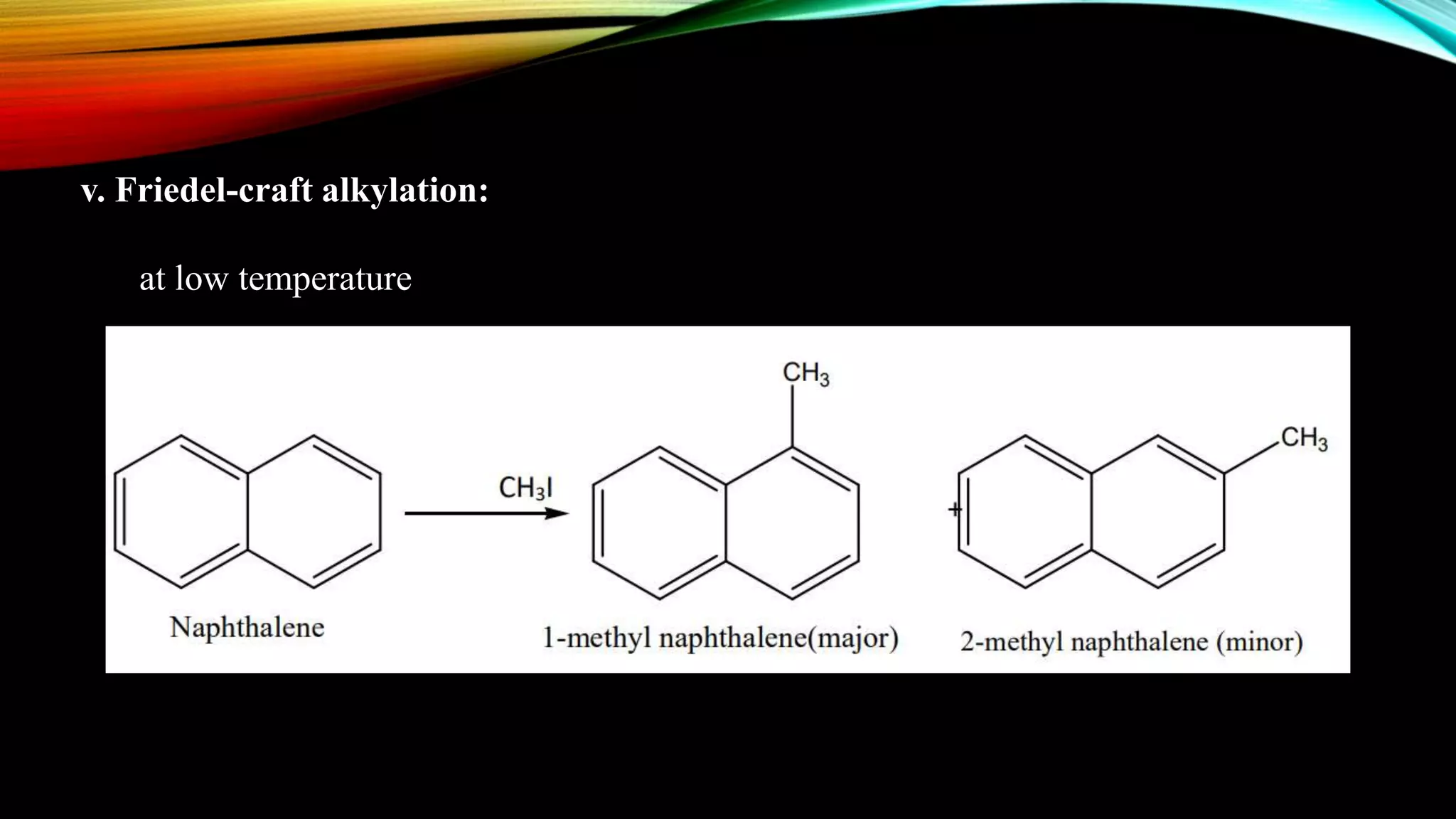
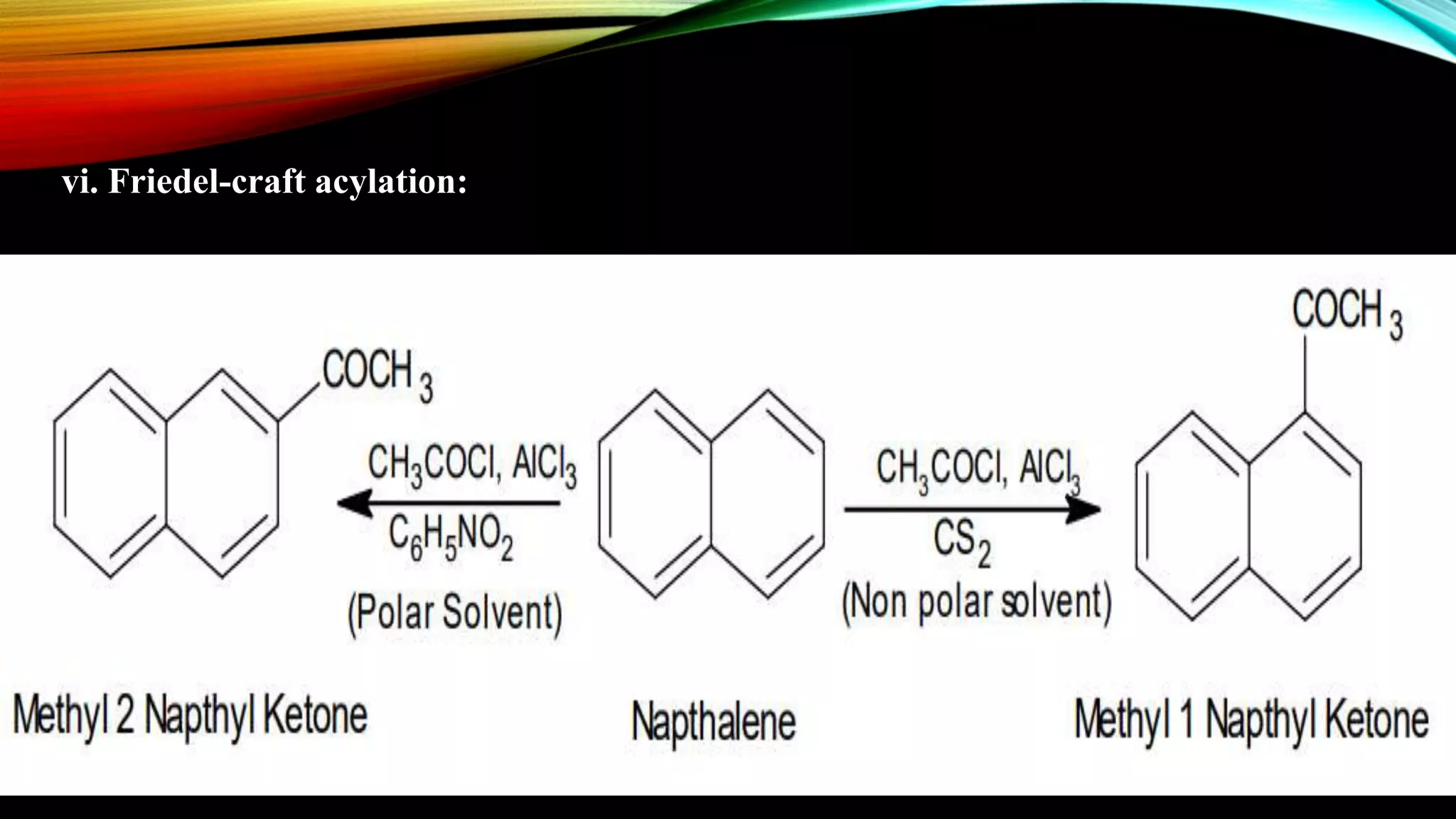
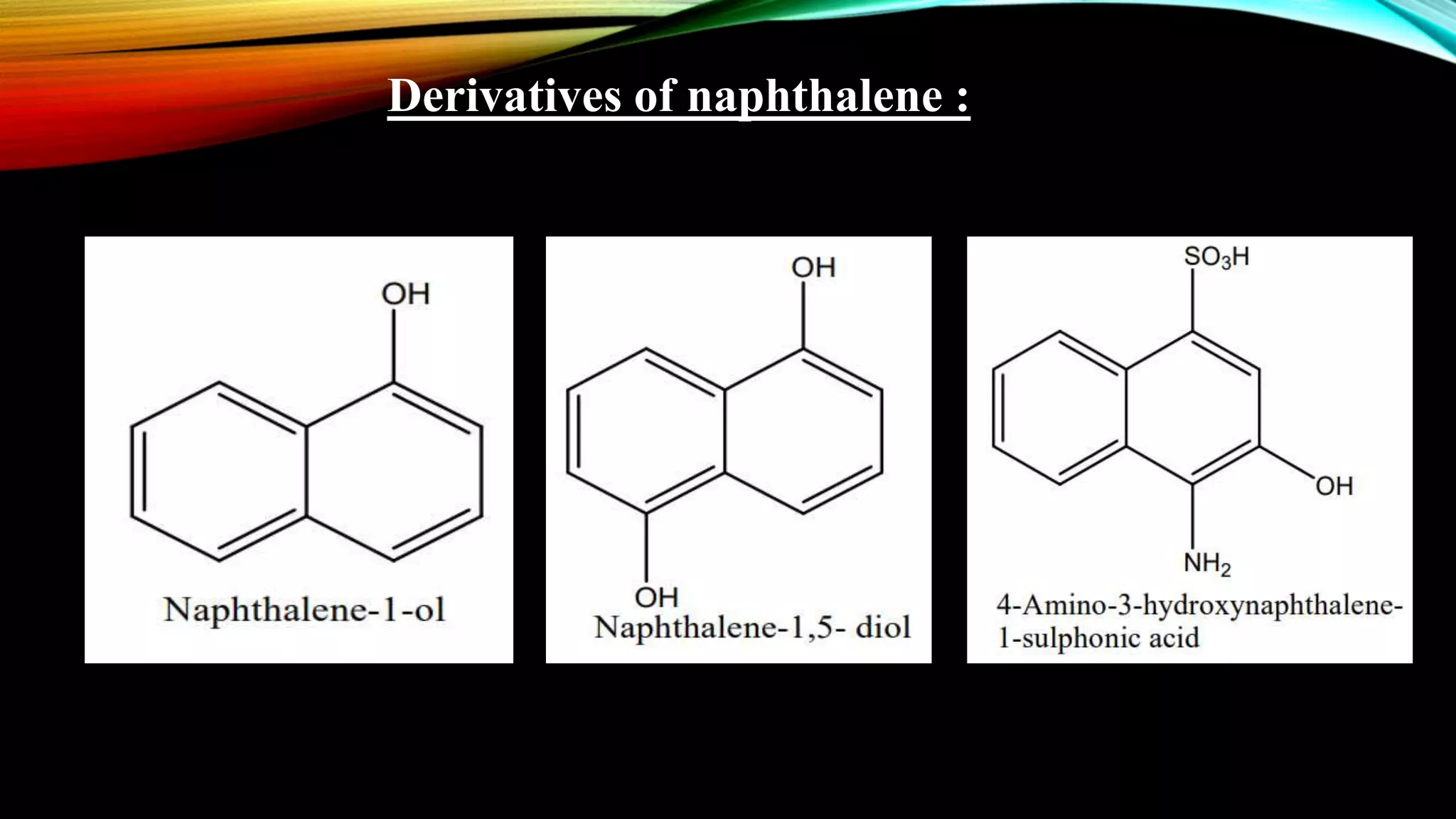
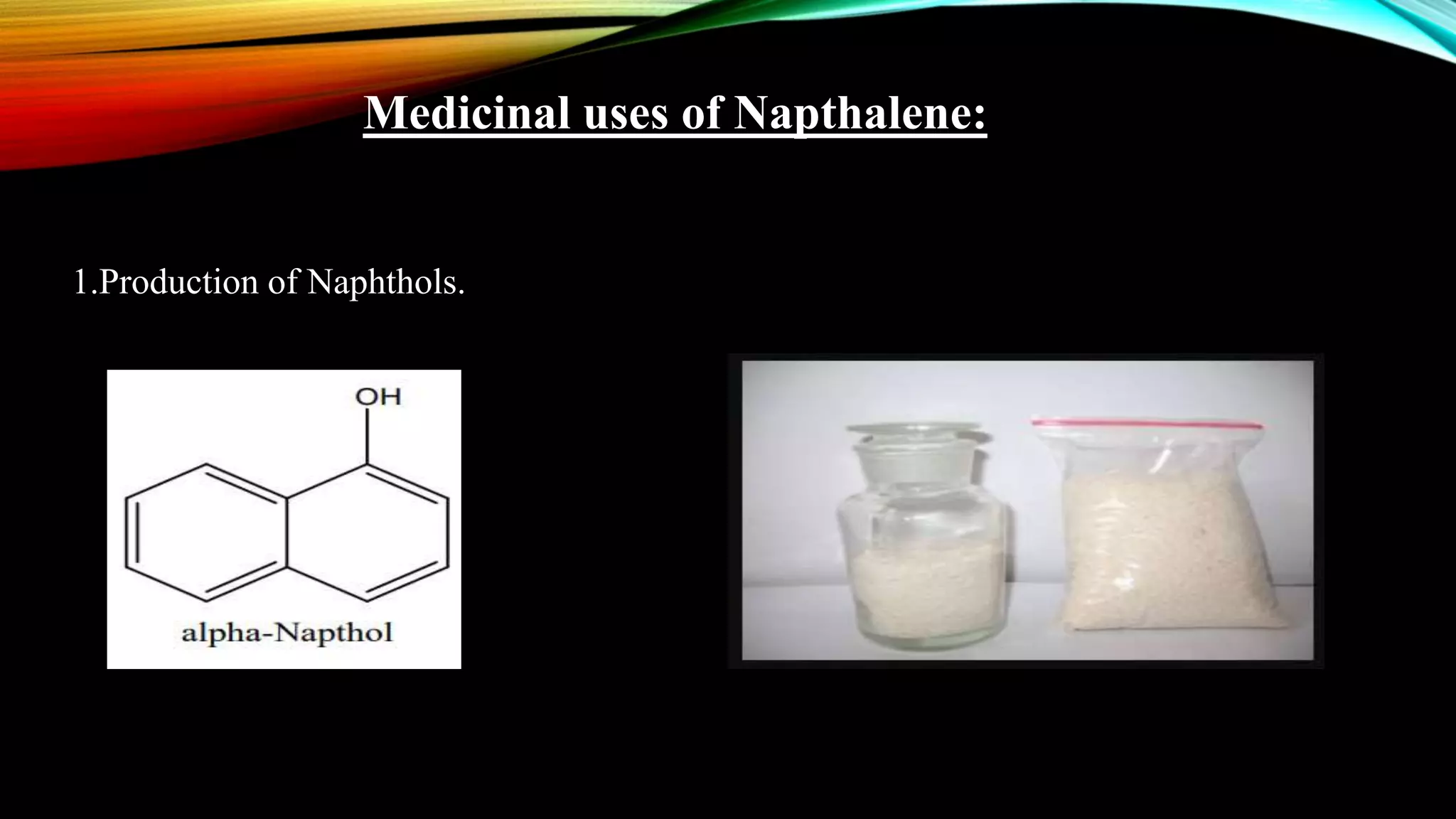
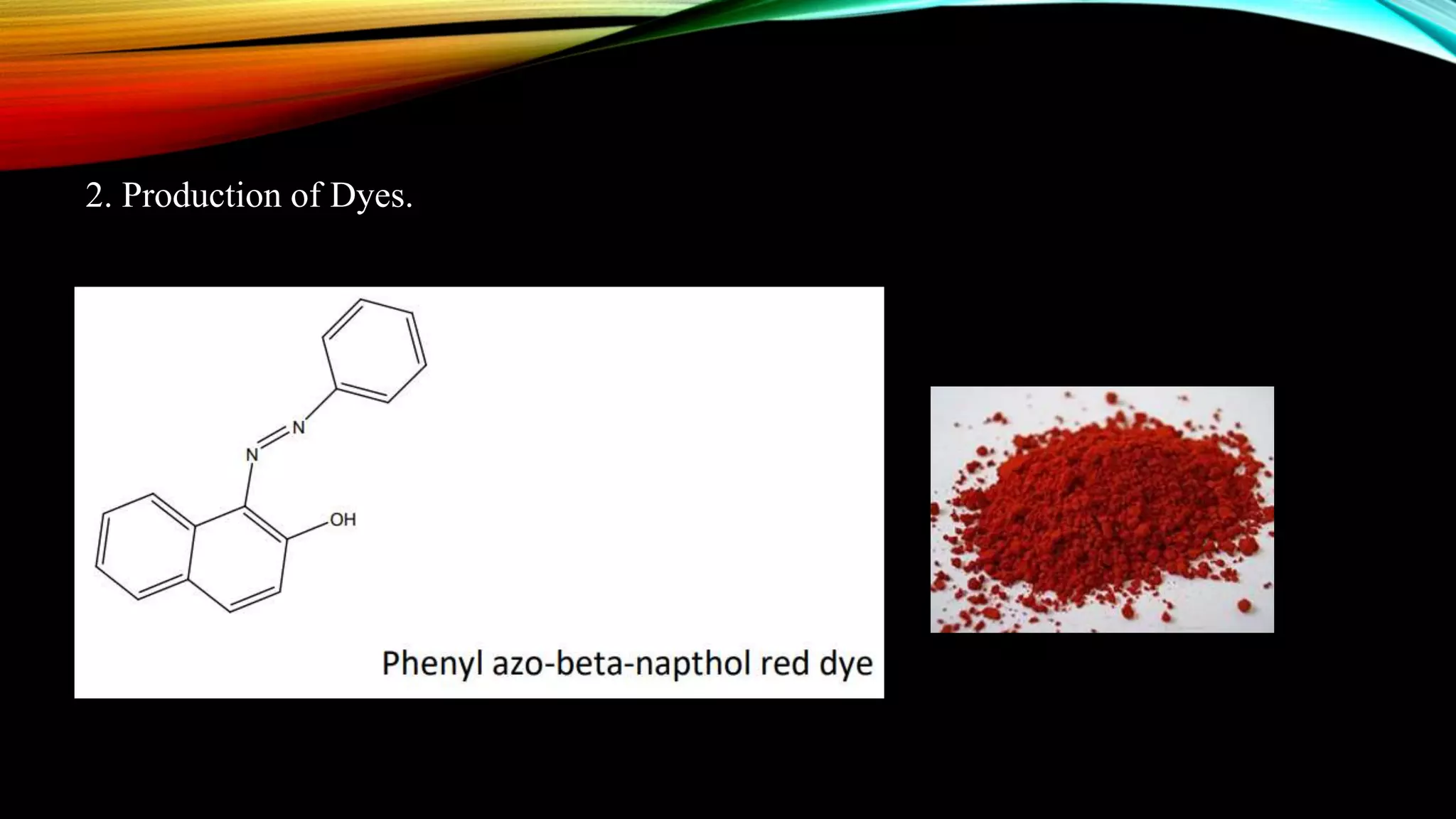
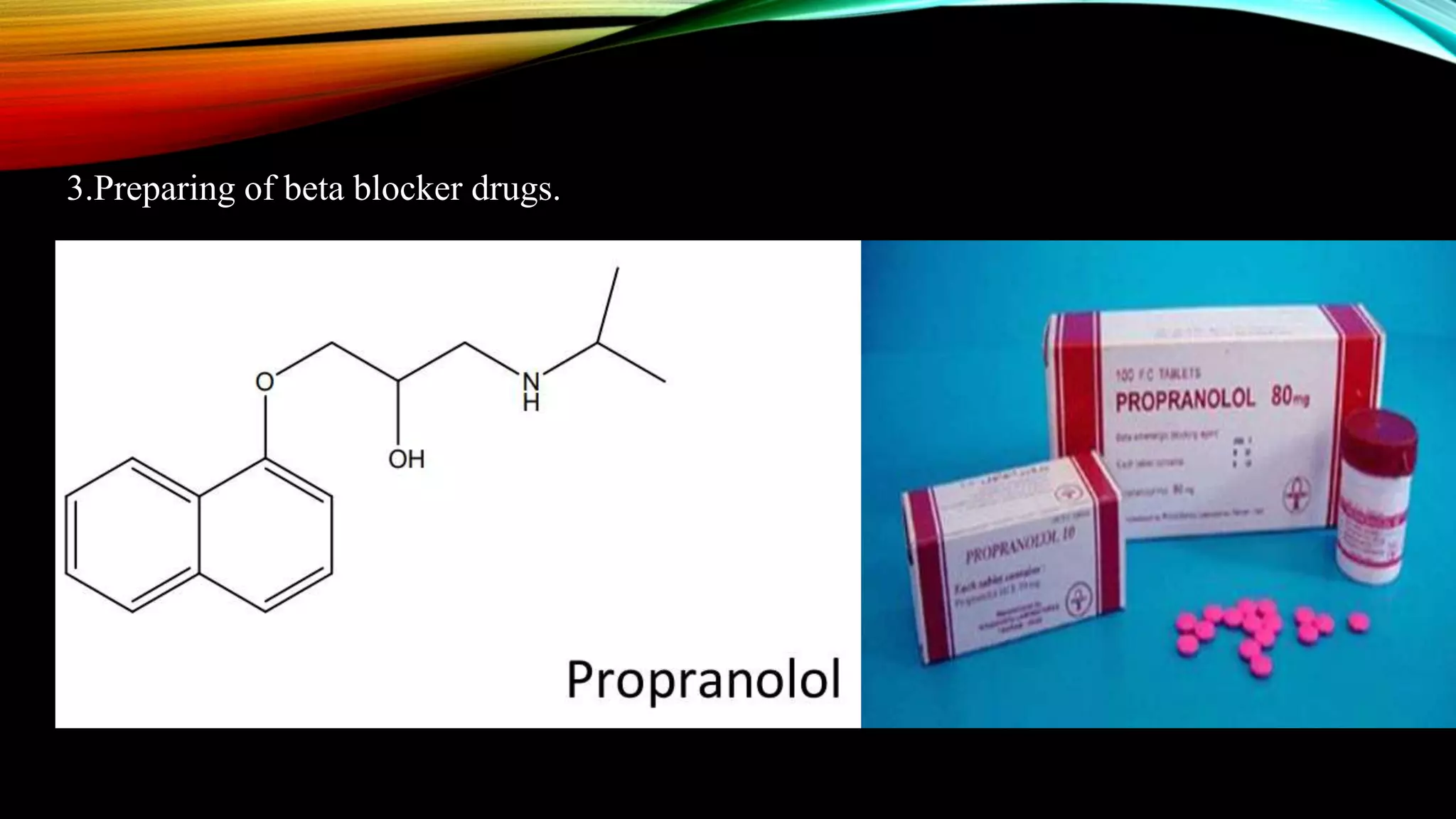
Naphthalene is an aromatic hydrocarbon consisting of two fused benzene rings. It is isolated from coal tar, where it makes up 6-10% of the fraction. Naphthalene crystallizes as white plates with a strong odor. It sublimes readily and is insoluble in water but soluble in organic solvents. Naphthalene undergoes substitution and addition reactions similarly to benzene, though it is somewhat less aromatic. Resonance structures show that the C1-C2 bond has more double bond character, making it shorter than the C2-C3 bond. Naphthalene has several industrial and medical uses including in the production of naphthols, dyes, beta blocker drugs