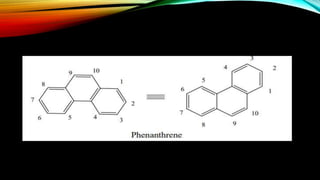
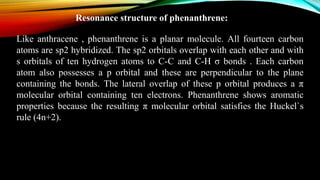
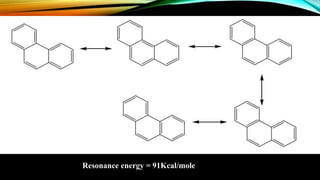
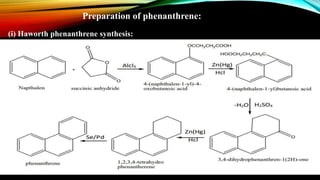
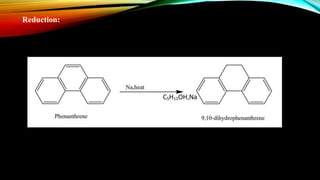
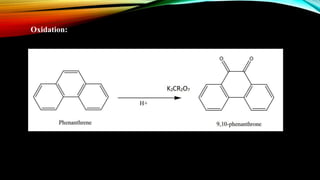
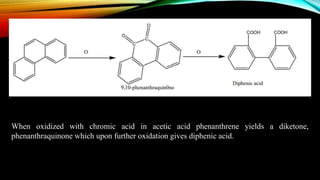
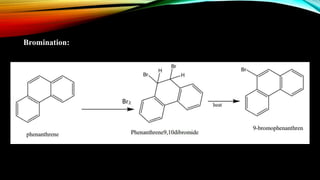
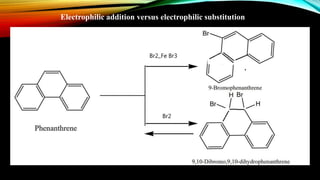
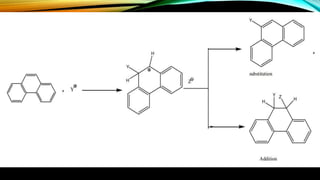
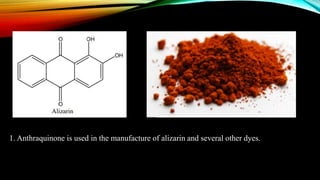
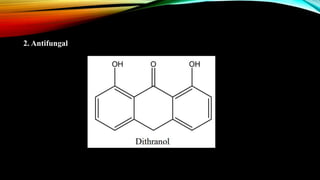
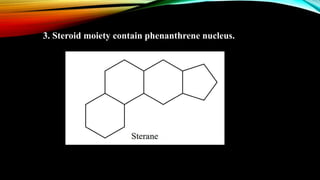
Phenanthrene is a polycyclic aromatic hydrocarbon composed of three fused benzene rings. It appears as a white powder with blue fluorescence. Phenanthrene shows aromatic properties and is the backbone molecule for morphine. It undergoes reactions like oxidation, reduction, bromination, and electrophilic addition or substitution. Derivatives of phenanthrene like papaverine are used as vasodilators to increase blood flow. Phenanthrene and its derivatives have several medicinal uses including manufacturing dyes, acting as antifungals, and being components of steroids, sex hormones, bile acids, and cardiac glycosides.