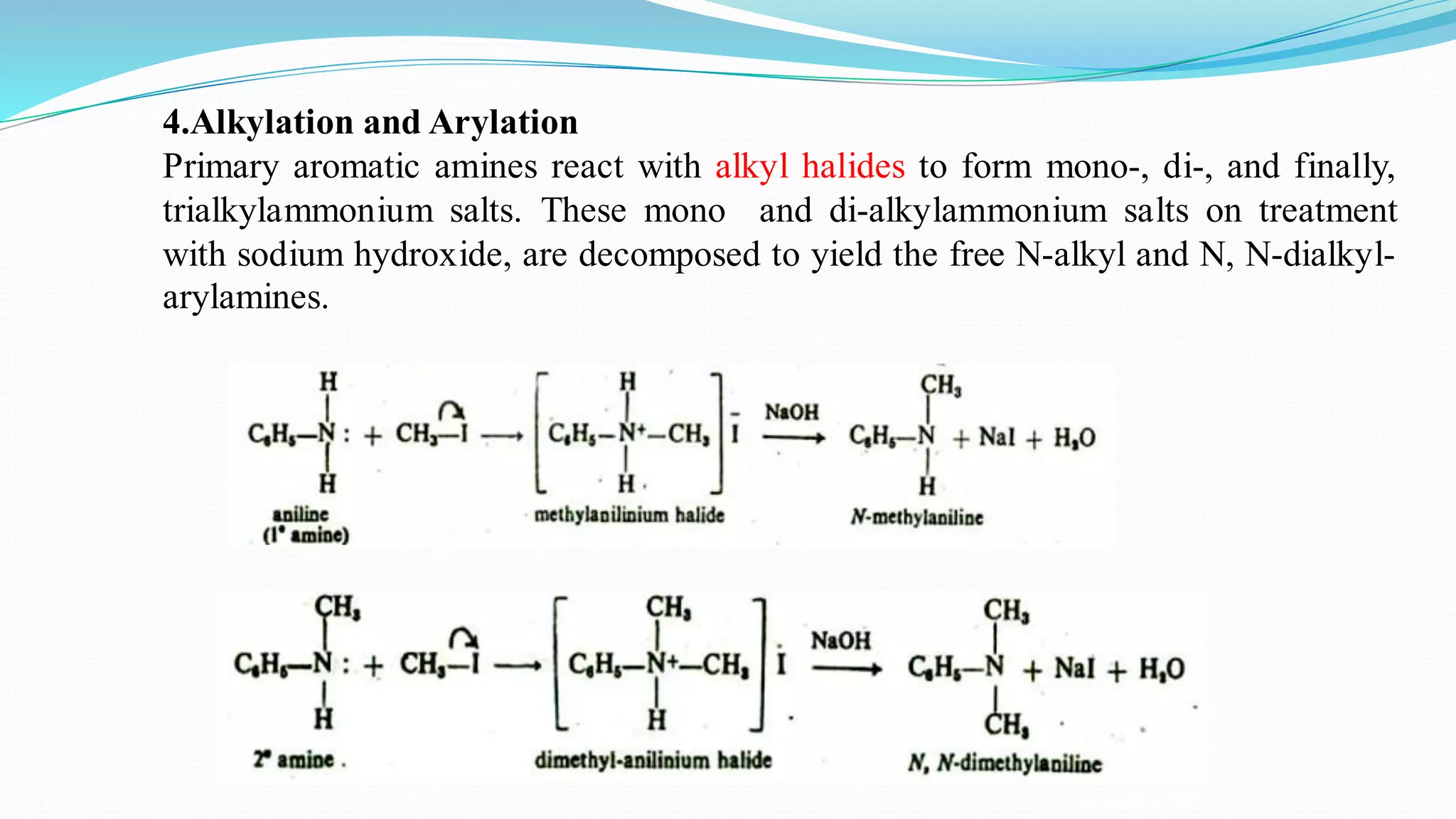
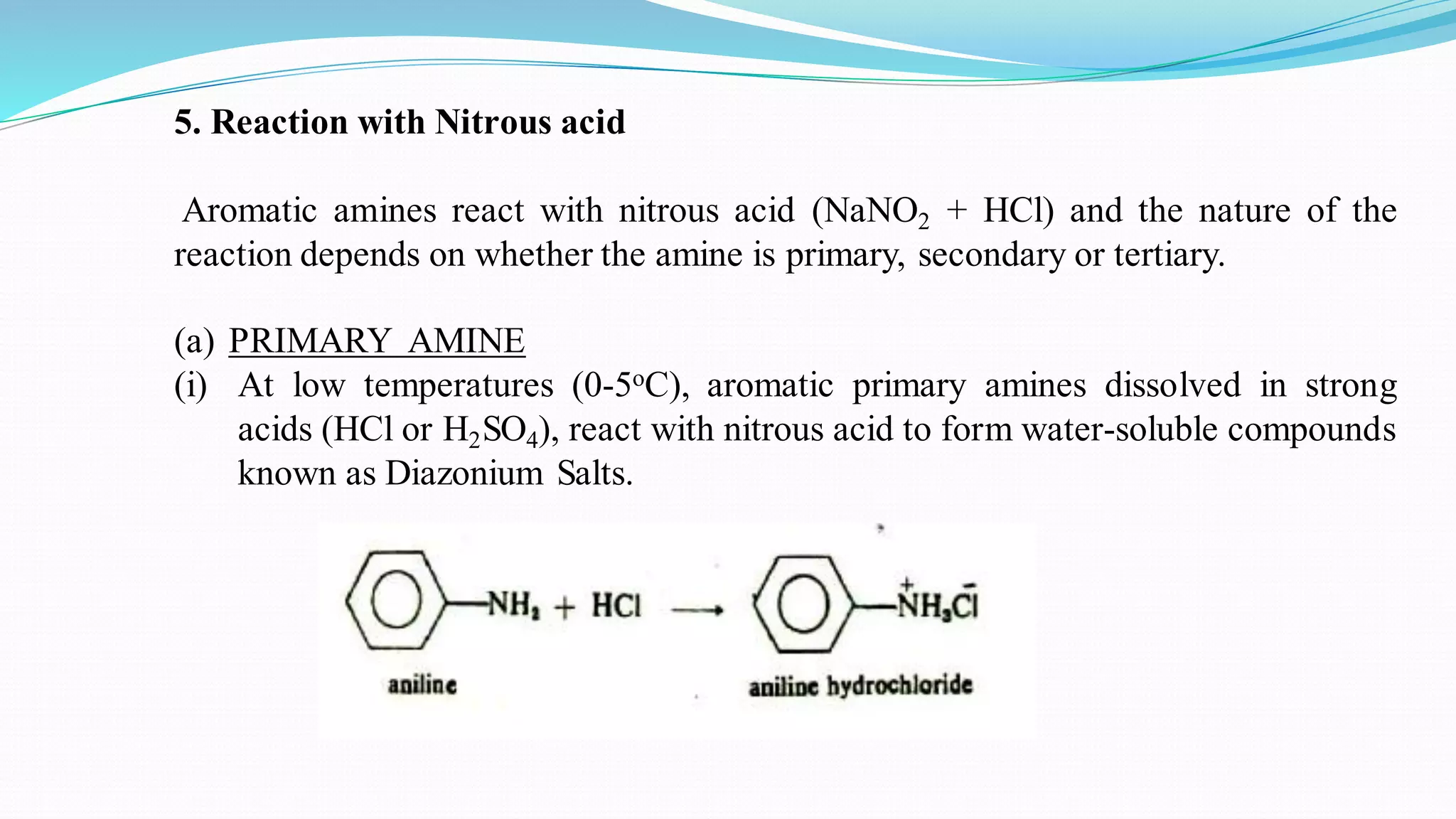
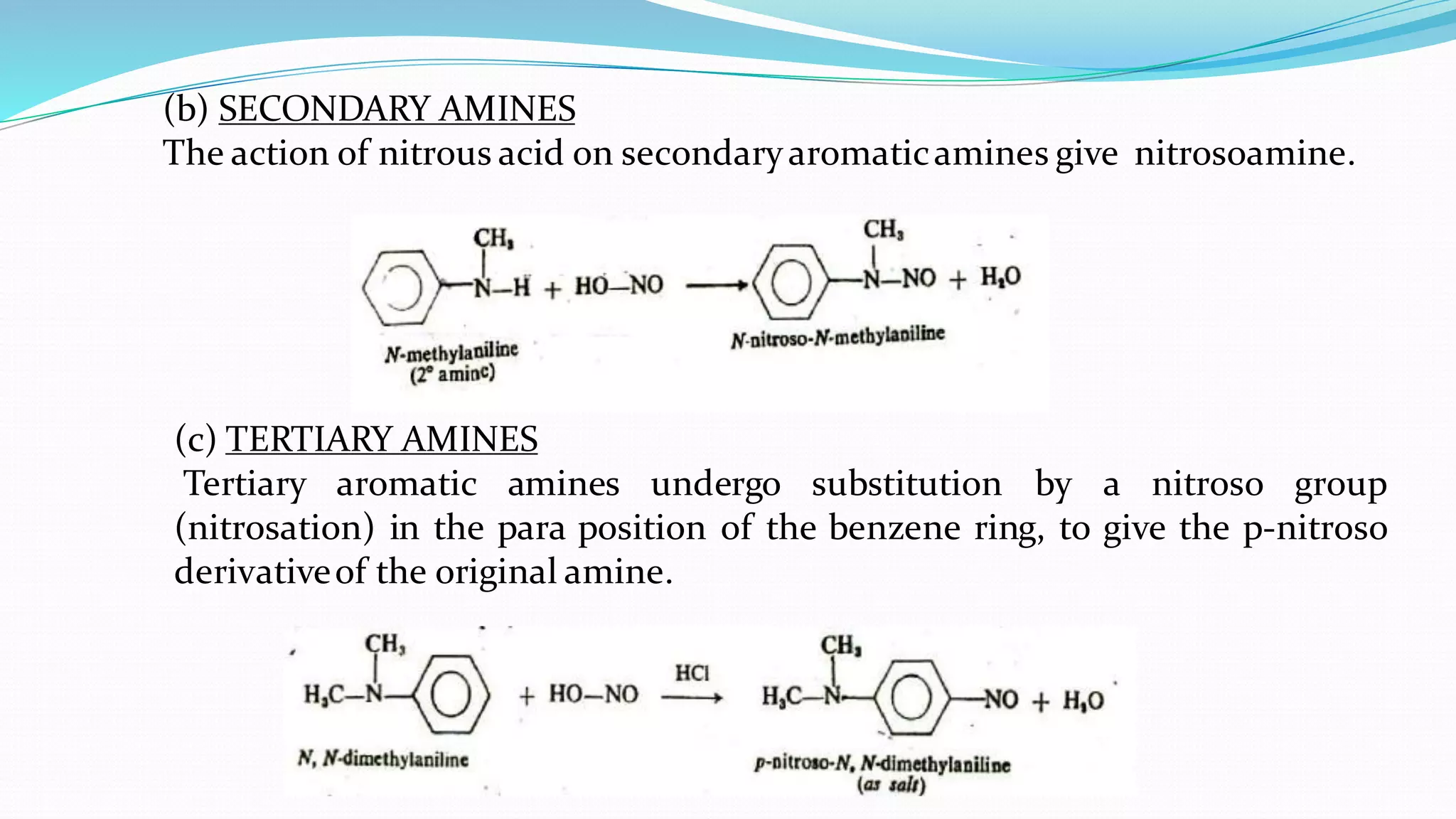
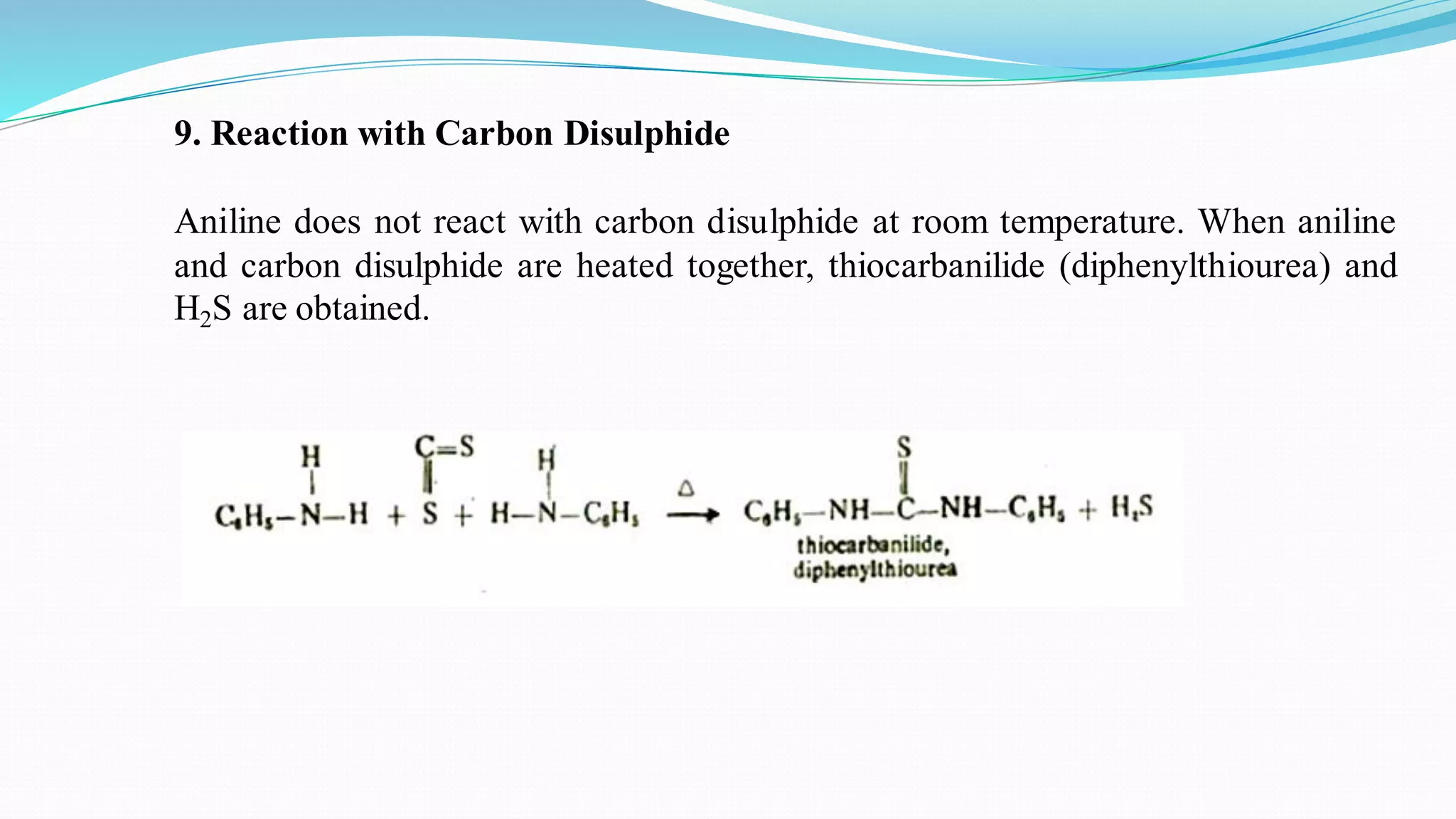
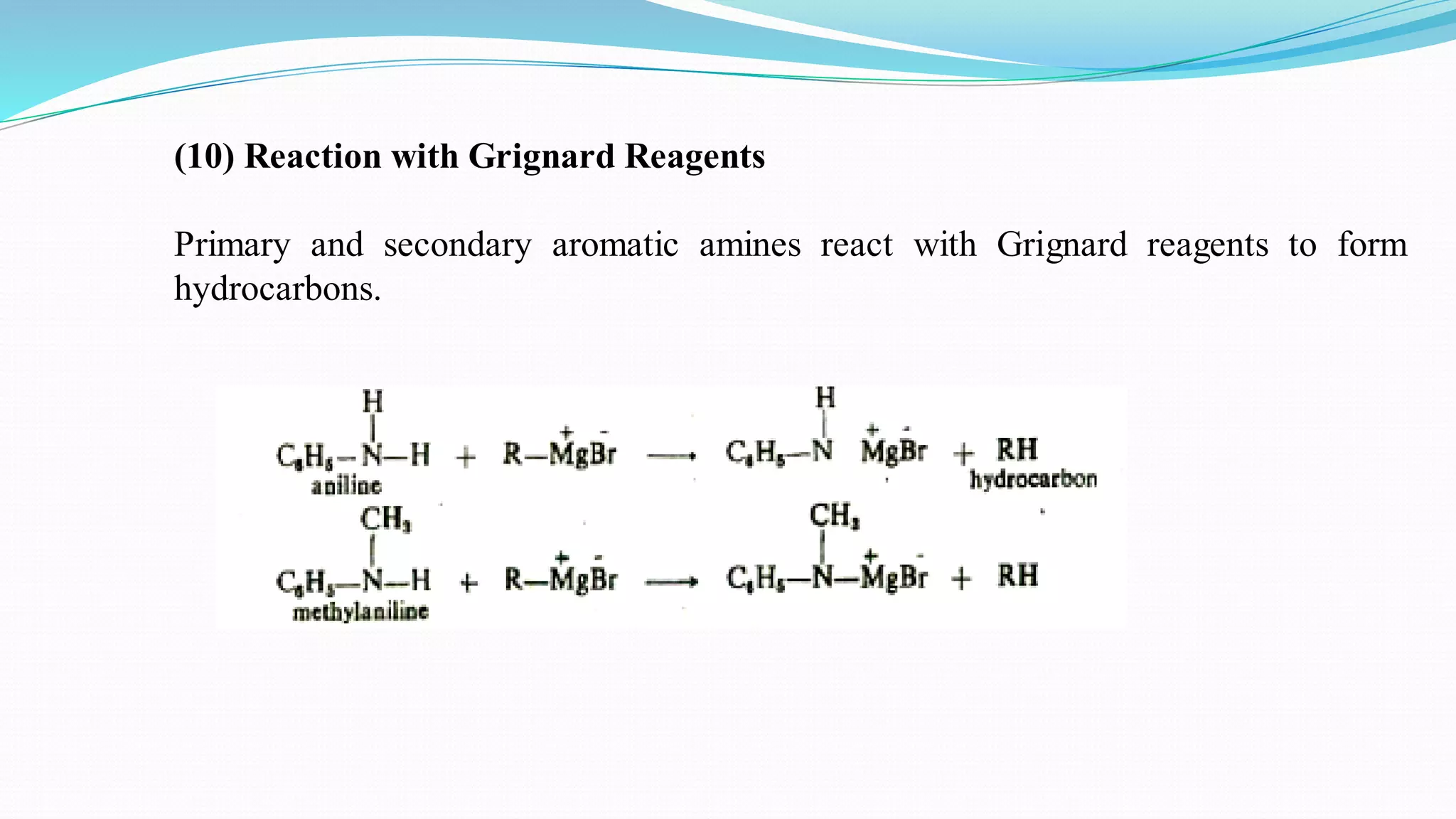
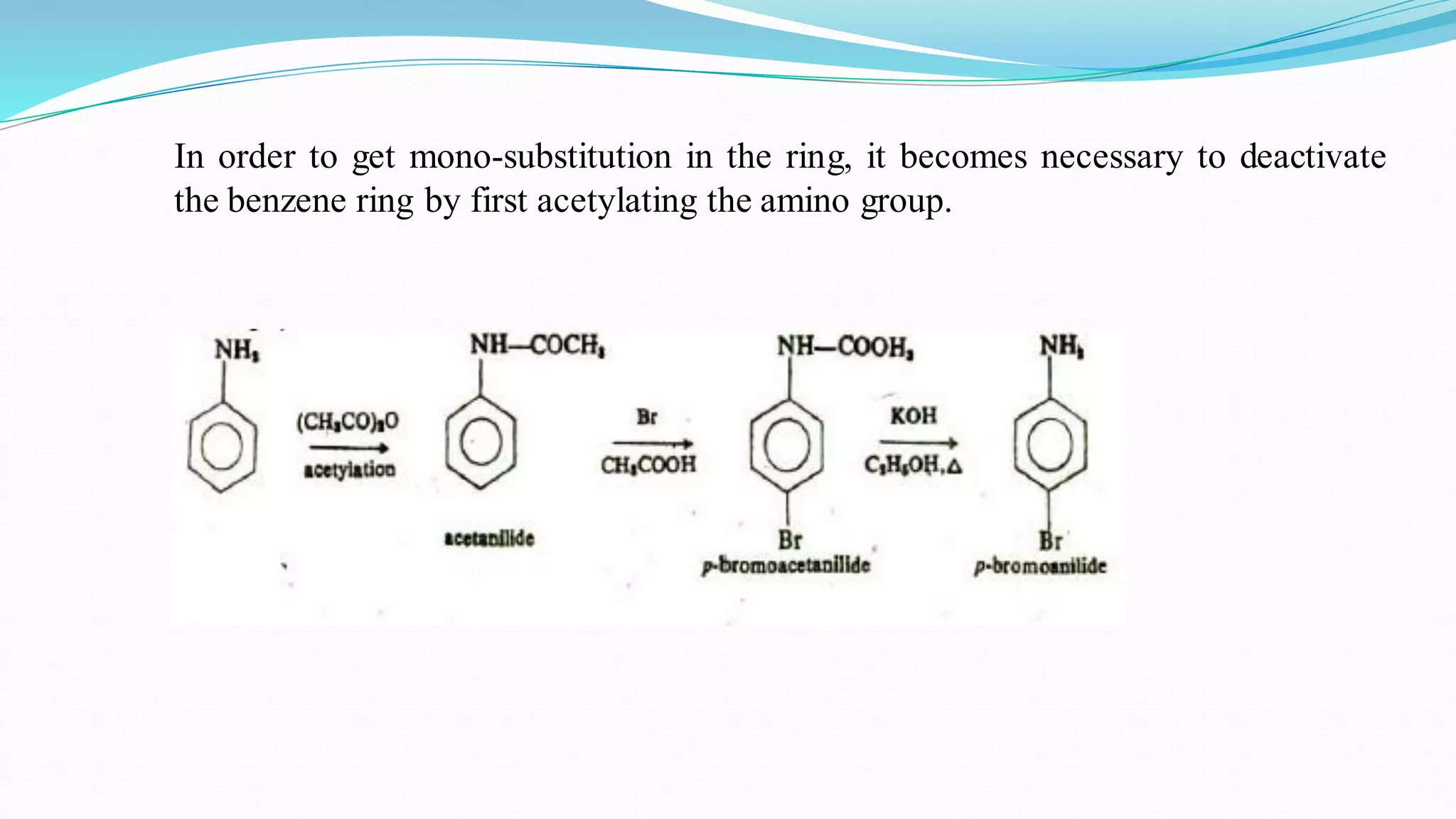
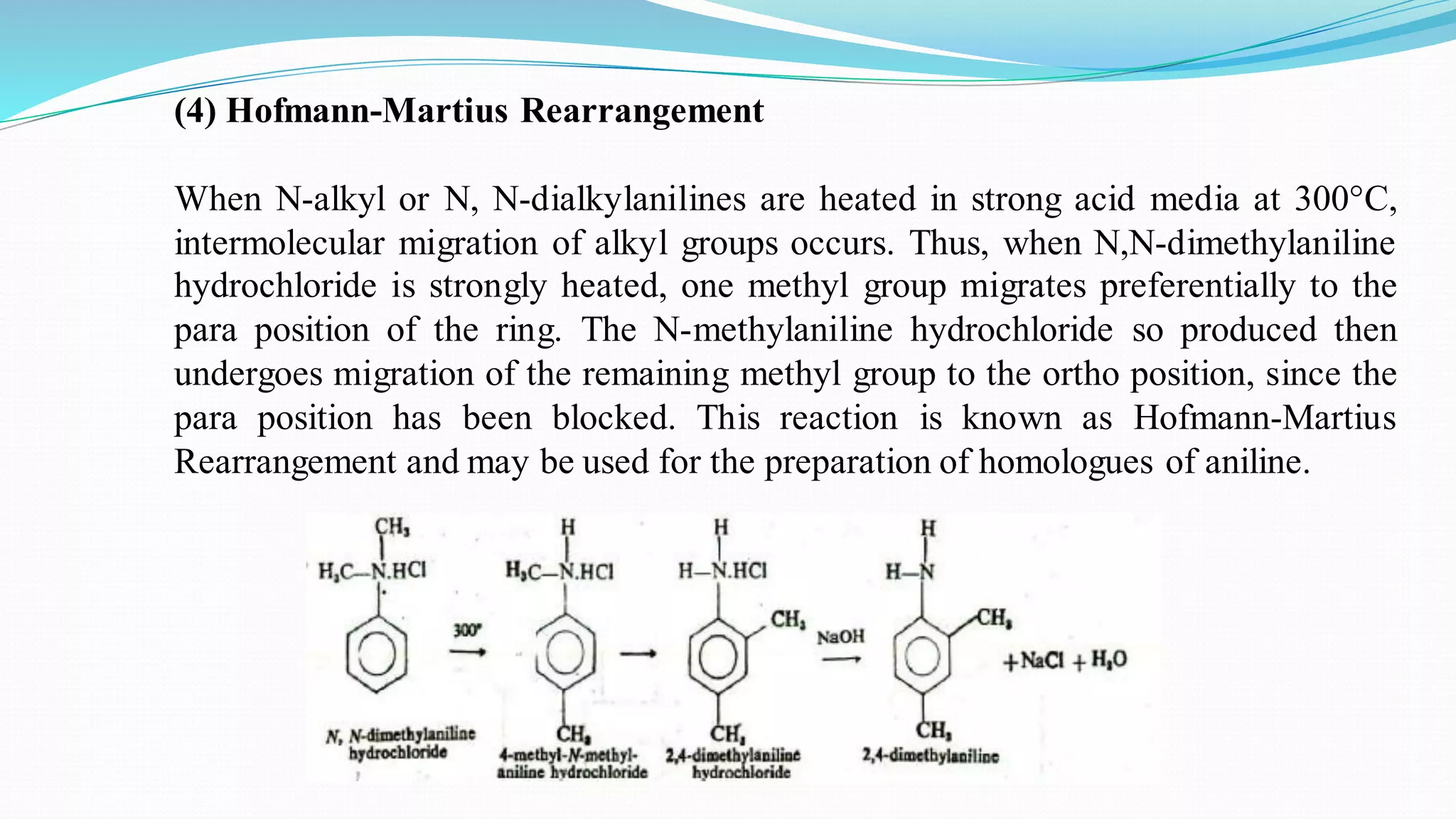
This document summarizes various reactions of the -NH2 group and benzene ring in aromatic amines. Reactions of the -NH2 group include salt formation, acylation, sulphonylation, alkylation, arylation, reaction with nitrous acid, oxidation, reaction with aldehydes, carbylamine reaction, and reaction with carbon disulfide and Grignard reagents. Reactions involving the benzene ring include bromination, nitration, sulphonation, and the Hofmann-Martius rearrangement. Specific examples are provided for many of the reaction types.