Embed presentation
Downloaded 178 times

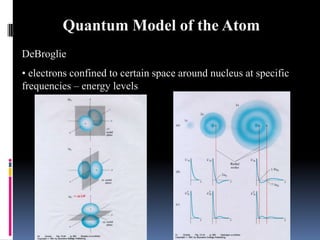
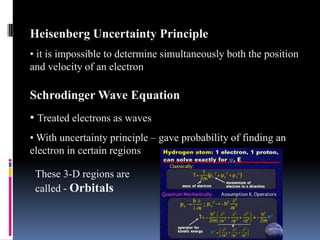
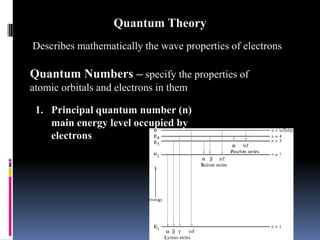

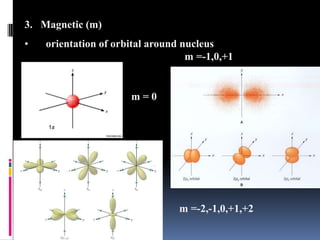
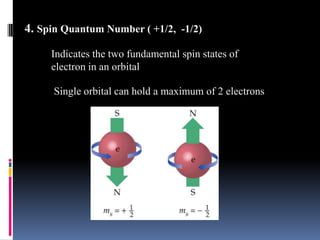

Louis de Broglie proposed that electrons behave as waves, confined to certain regions around the nucleus at specific energy levels, known as orbitals. The Heisenberg Uncertainty Principle states that it is impossible to know both the position and momentum of an electron simultaneously. Schrodinger's wave equation treats electrons as waves and uses the uncertainty principle to describe the probability of finding electrons in certain orbital regions. Quantum numbers specify the properties of orbitals and electrons, including the principal quantum number for energy level, angular momentum quantum number for orbital shape, and magnetic quantum number for orbital orientation.








