The document discusses several topics related to the electronic structures of atoms and electromagnetic radiation:
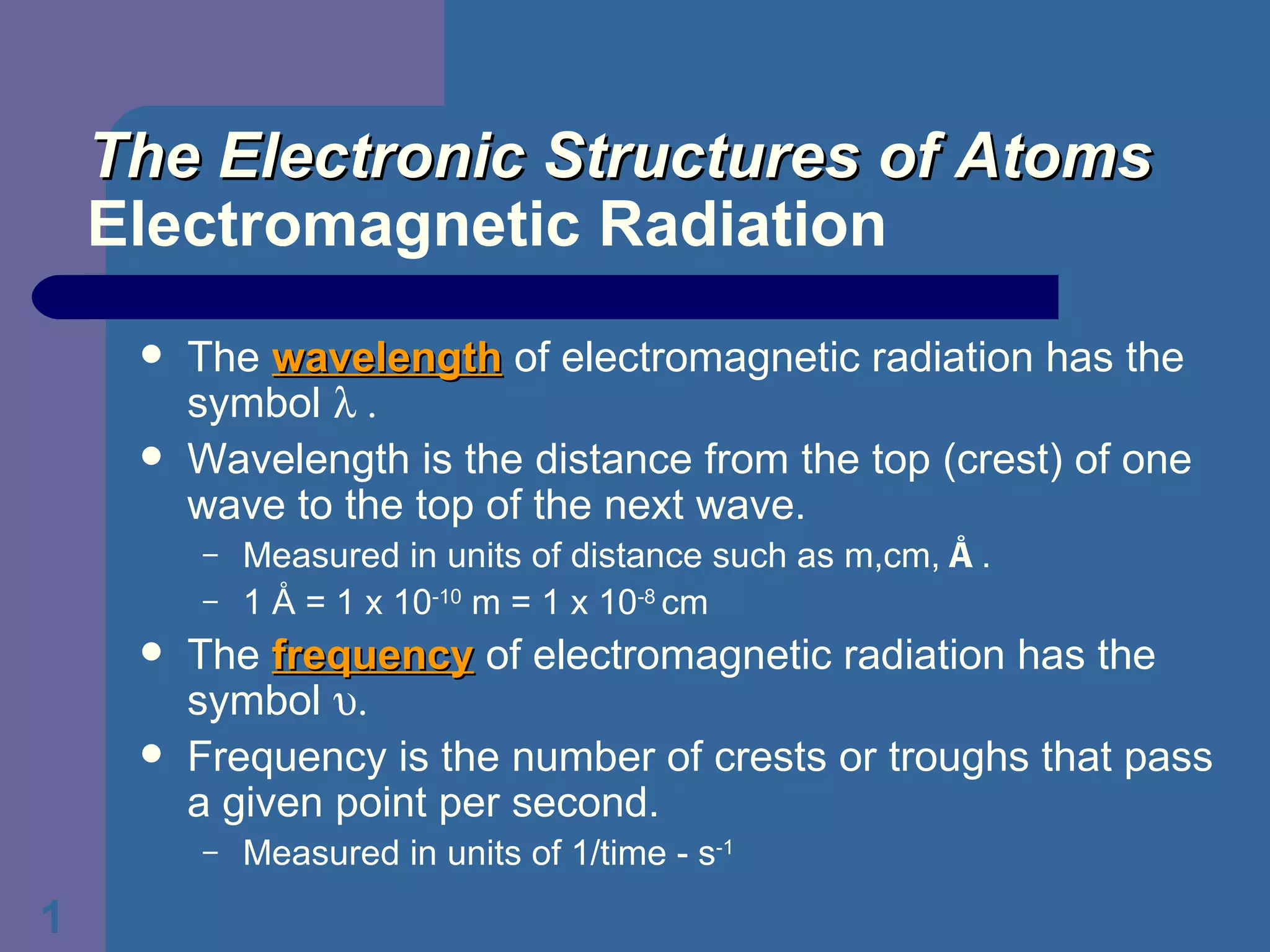
1. It defines wavelength and frequency of electromagnetic radiation and describes the relationship between them.
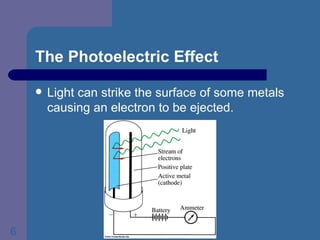
2. It discusses Max Planck's realization that energy is quantized and light has particle characteristics based on his study of blackbody radiation.
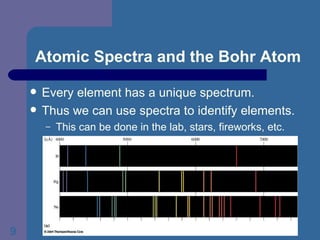
3. It explains Bohr's model of the hydrogen atom which incorporated Planck's quantum theory and correctly explained hydrogen's emission spectrum using discrete energy levels. However, the model failed for other elements.





















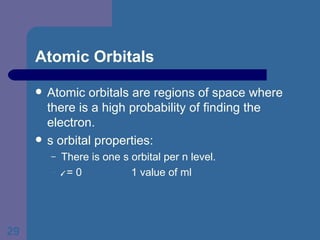
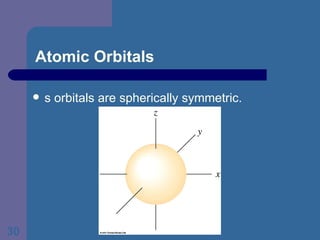

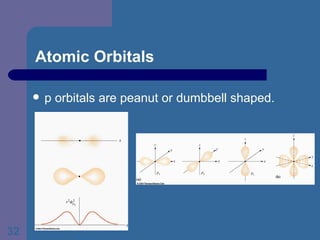

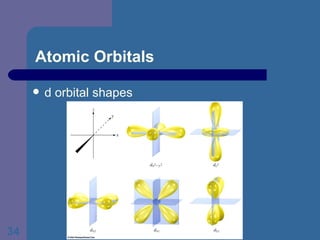







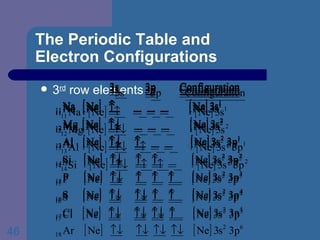
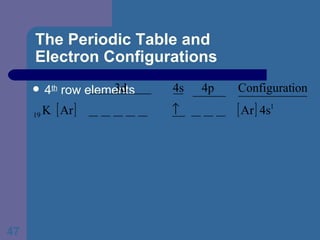
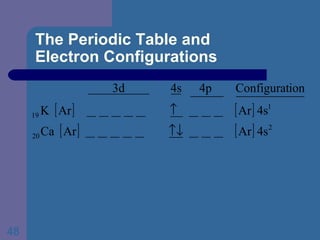
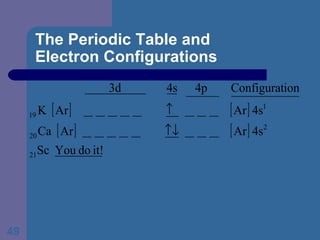
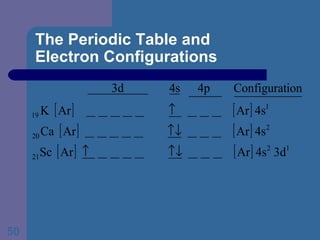
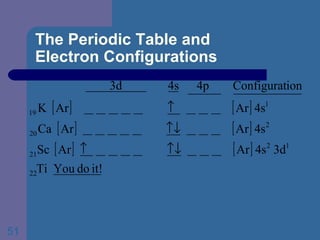
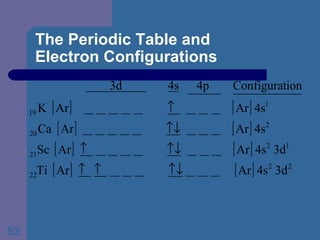
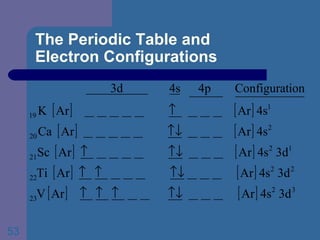
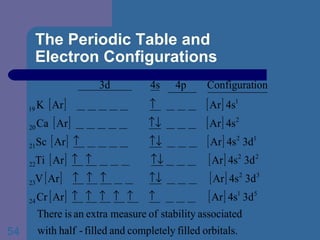

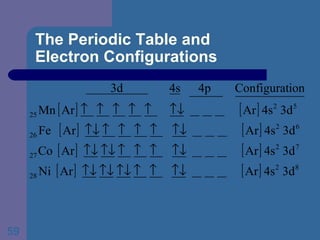
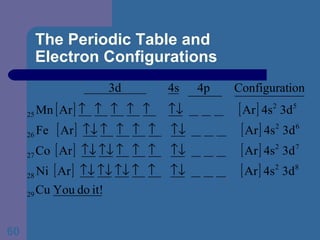
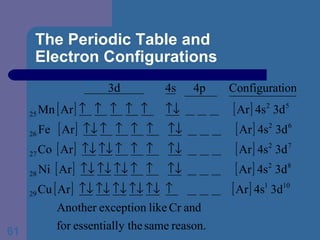
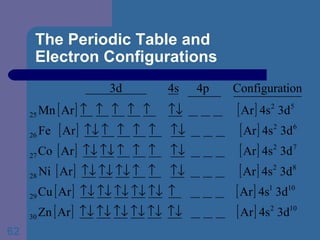
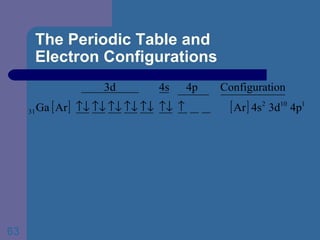
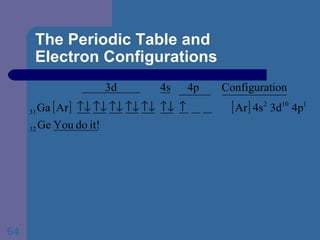
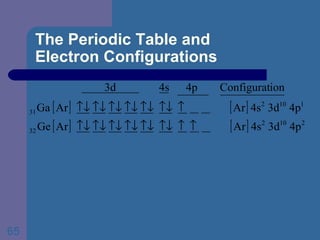
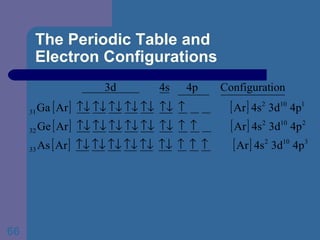
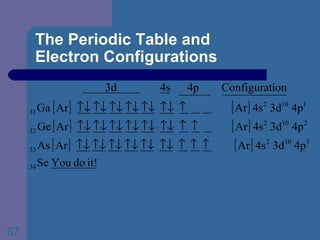
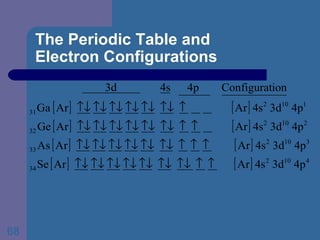
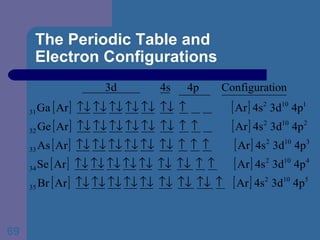
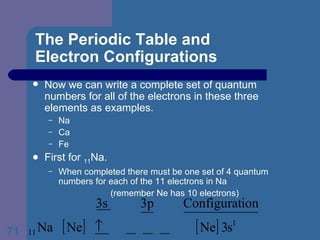
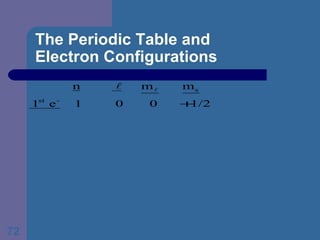
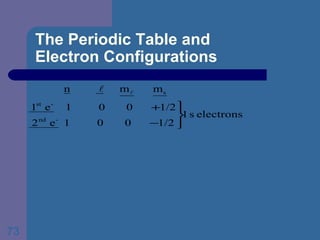
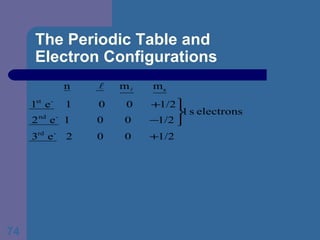
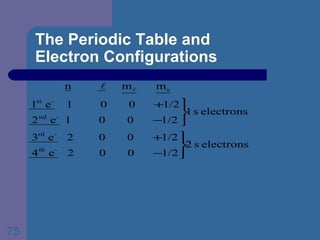
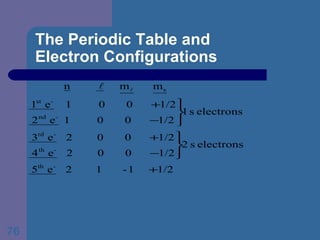
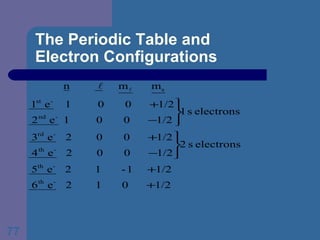
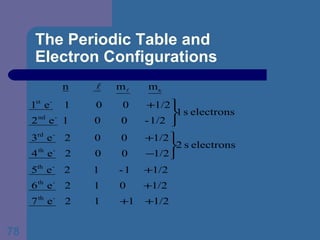
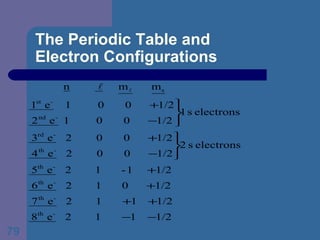
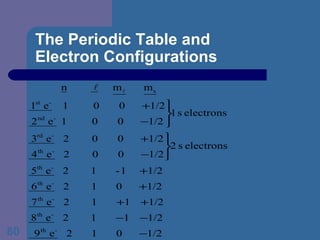
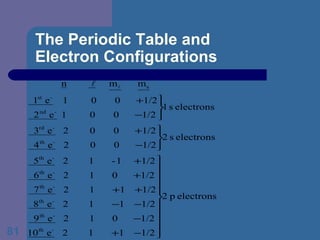
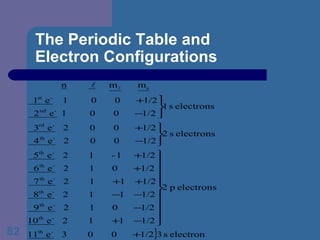
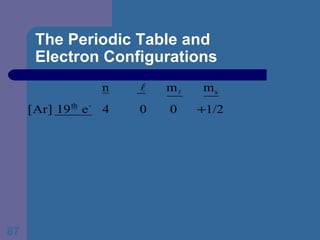
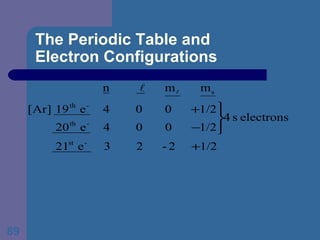
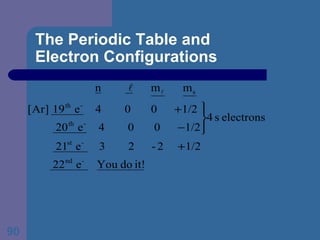
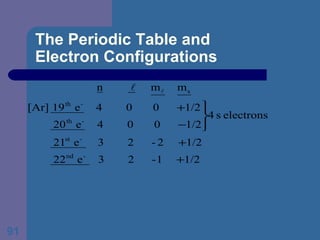
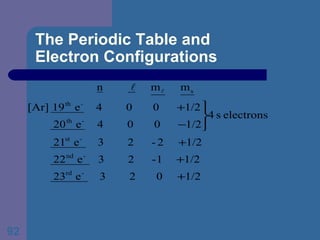
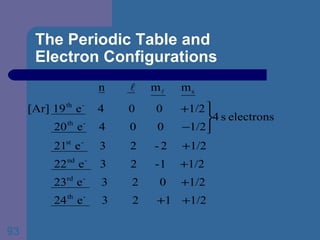
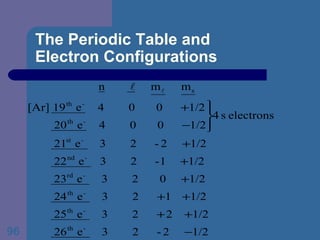
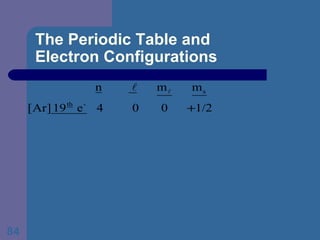
![The Periodic Table and Electron Configurations Next we will do the same exercise for 20 Ca. Again, when finished we must have one set of 4 quantum numbers for each of the 20 electrons in Ca. We represent the first 18 electrons in Ca with the symbol [Ar].](https://image.slidesharecdn.com/atom1-120113052252-phpapp02/85/Atom1-83-320.jpg)
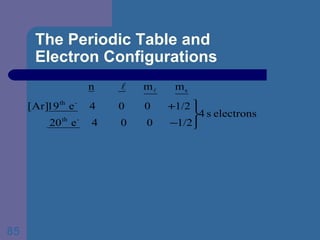
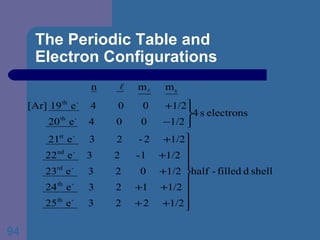
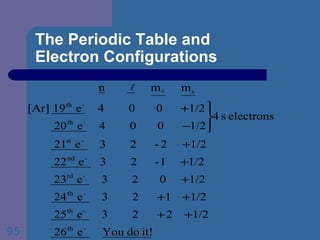
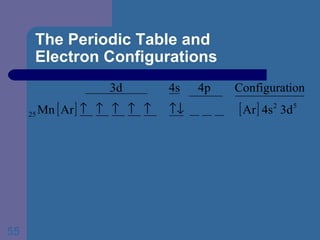
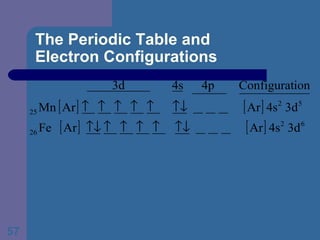
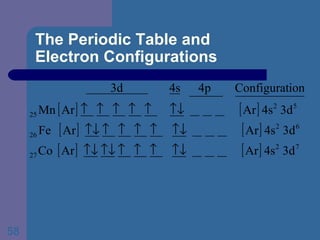
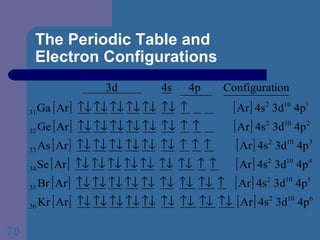
![The Periodic Table and Electron Configurations Finally, we do the same exercise for 26 Fe. We should have one set of 4 quantum numbers for each of the 26 electrons in Fe. To save time and space, we use the symbol [Ar] to represent the first 18 electrons in Fe](https://image.slidesharecdn.com/atom1-120113052252-phpapp02/85/Atom1-86-320.jpg)