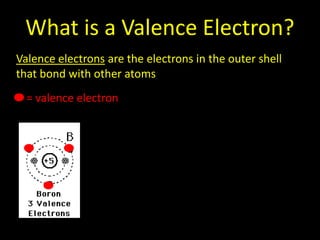
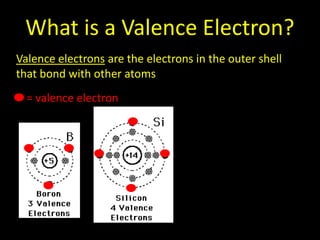
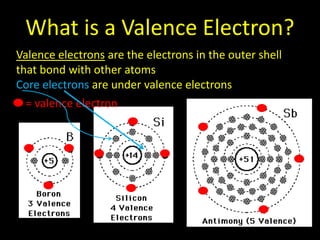
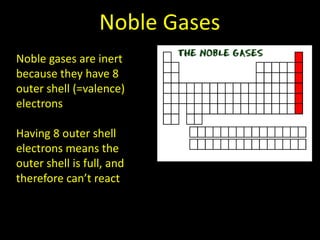
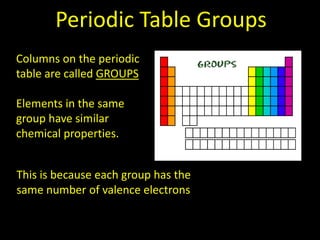
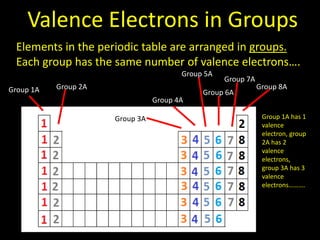
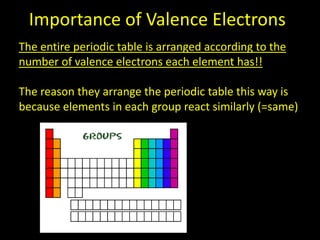
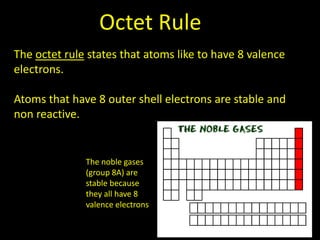
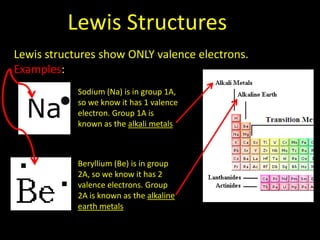
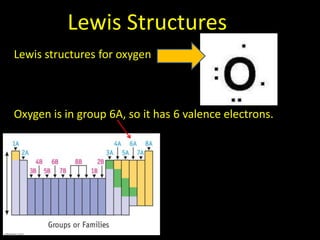
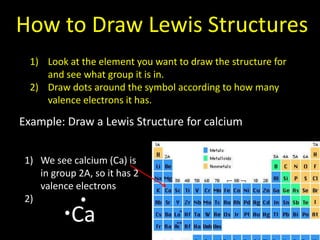
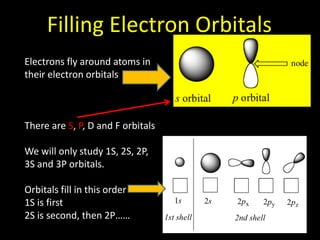
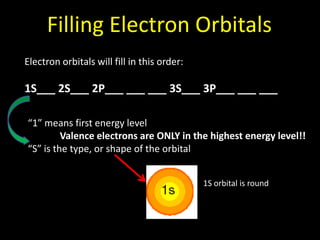
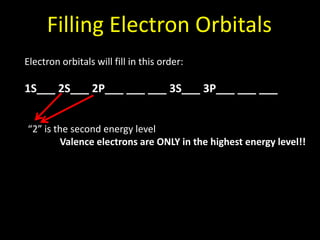
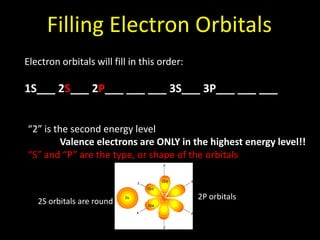
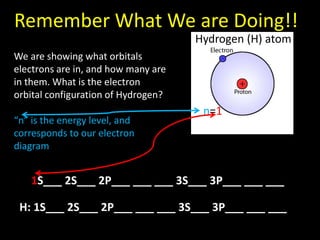
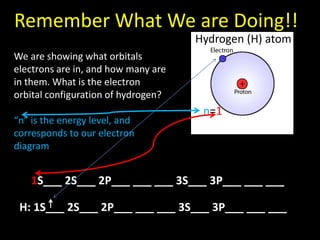
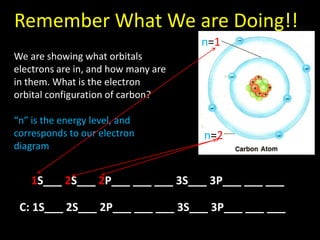
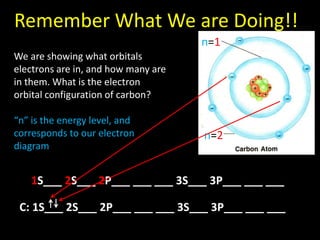
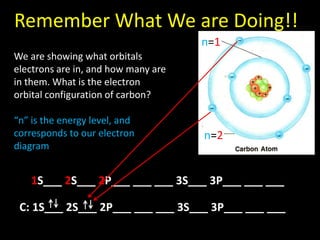
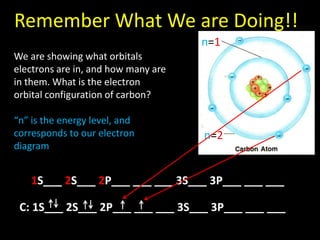
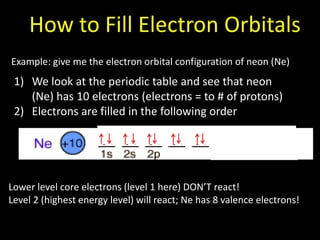
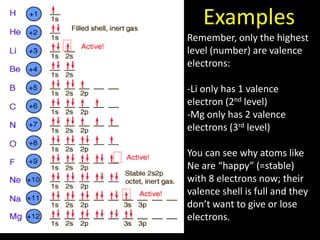
Valence electrons are the outermost shell electrons of an atom that are involved in bonding. Elements in the same group on the periodic table have the same number of valence electrons because they exhibit similar chemical properties based on their valence electron configuration. Atoms seek to attain a full outer shell of 8 electrons to achieve stability through gaining, losing or sharing valence electrons in chemical bonds.