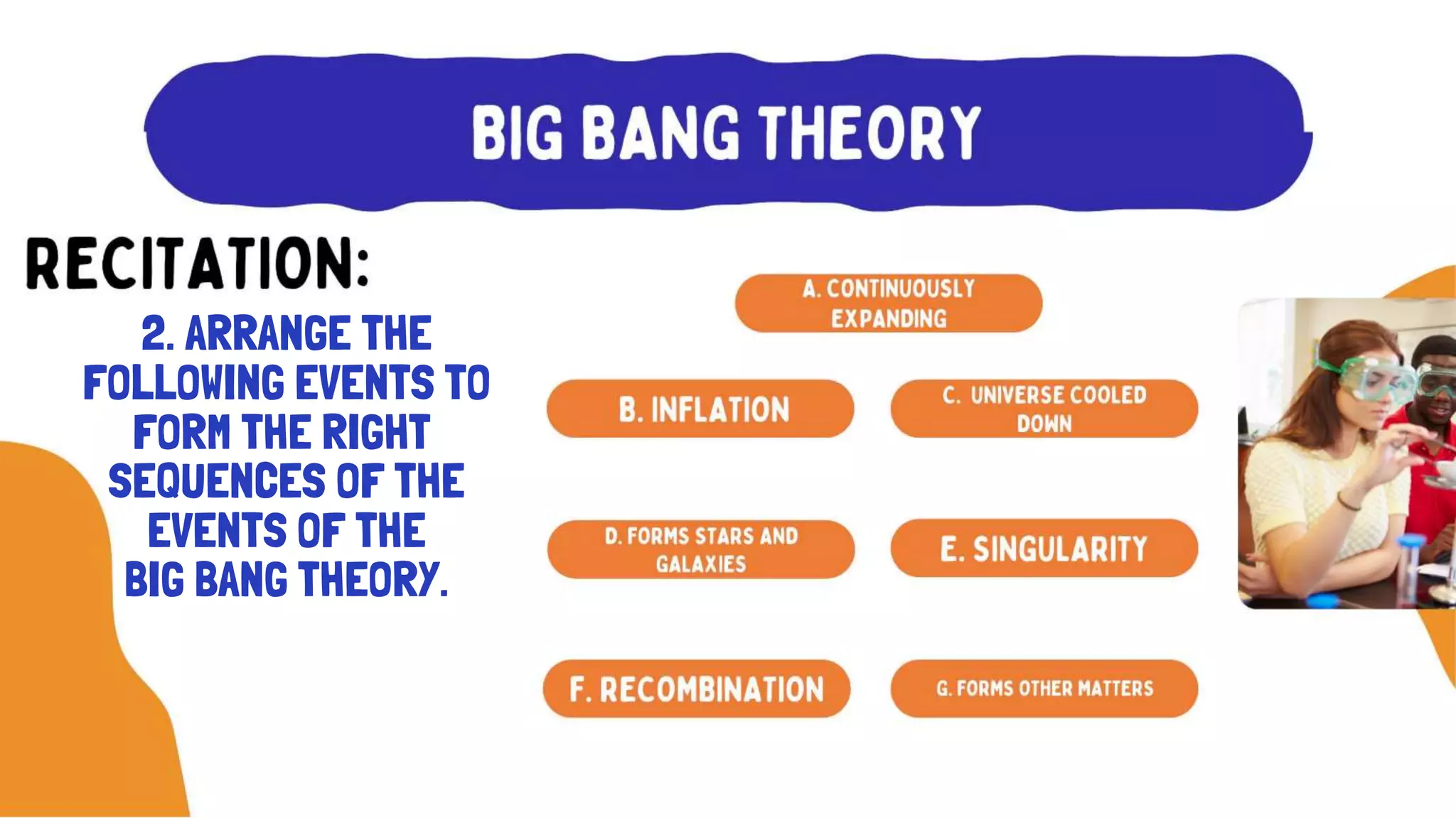
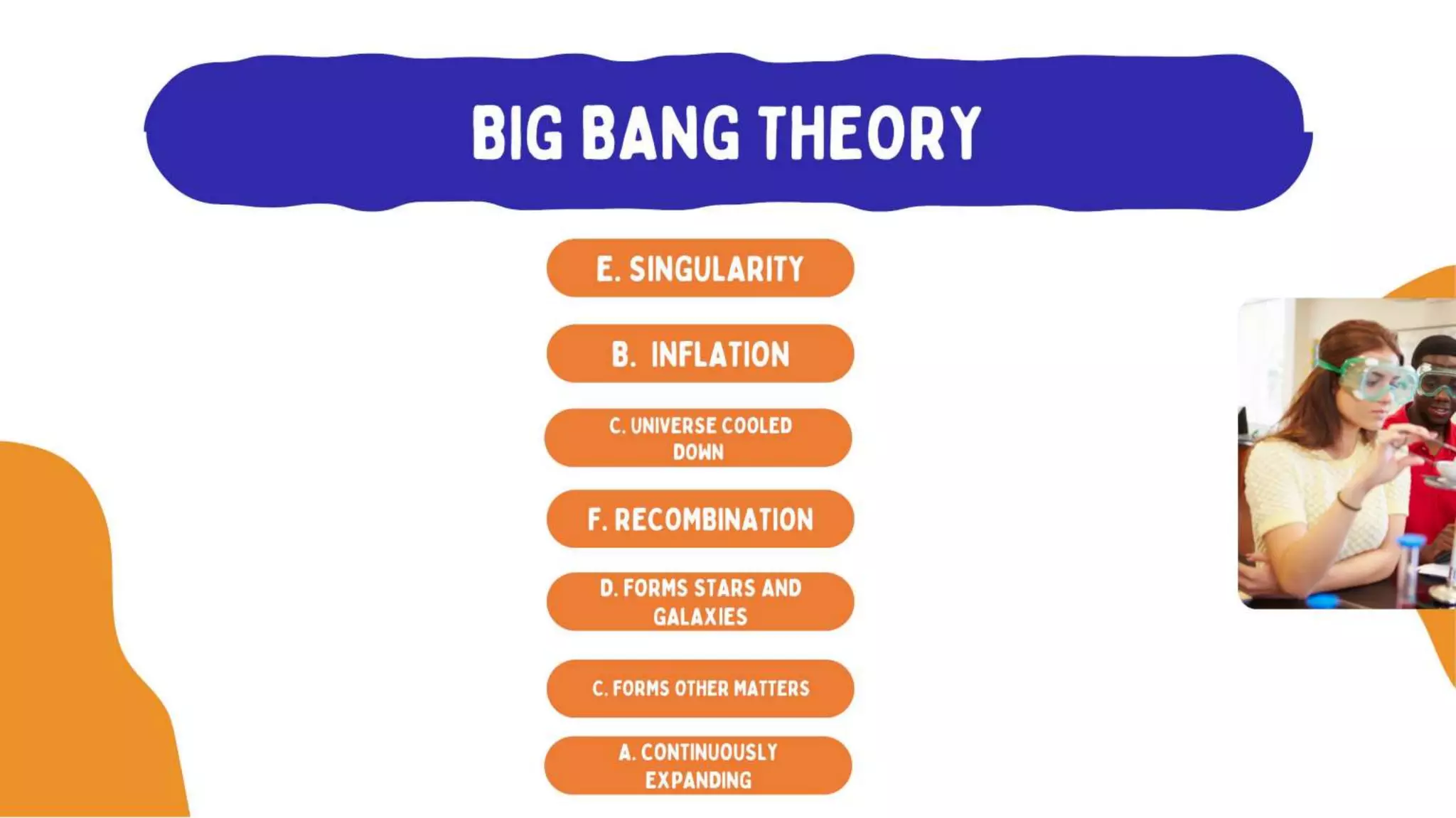
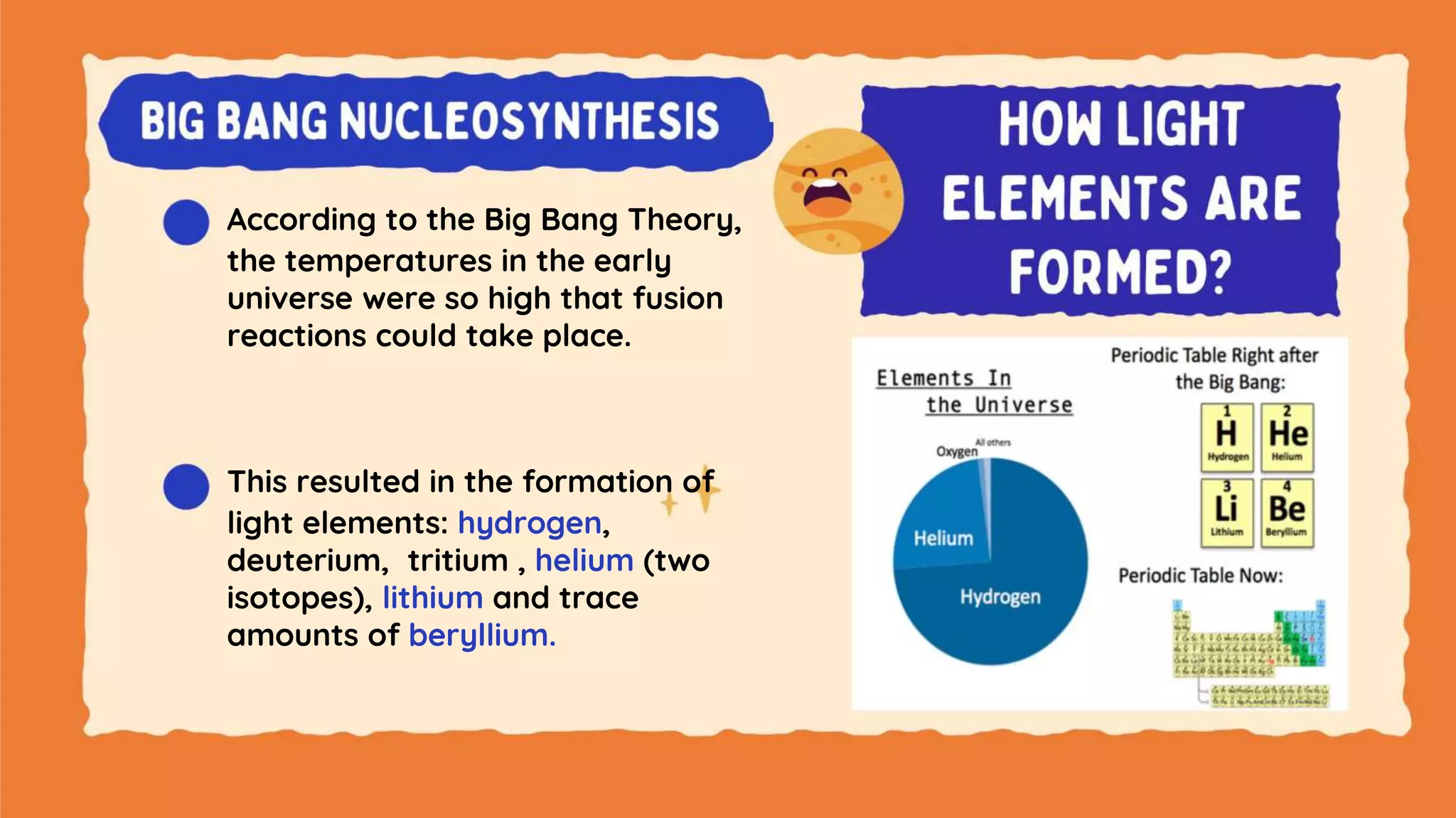
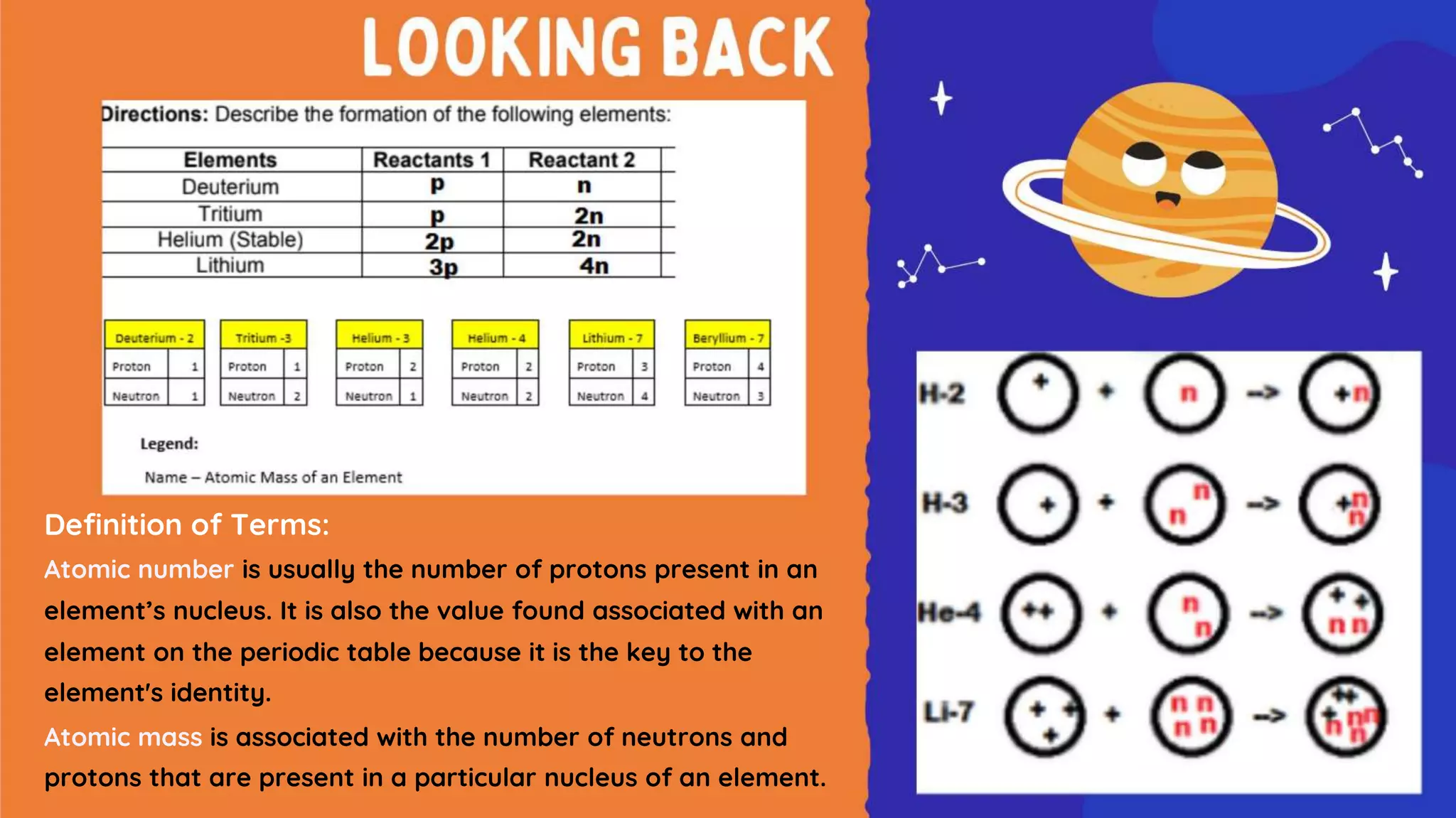
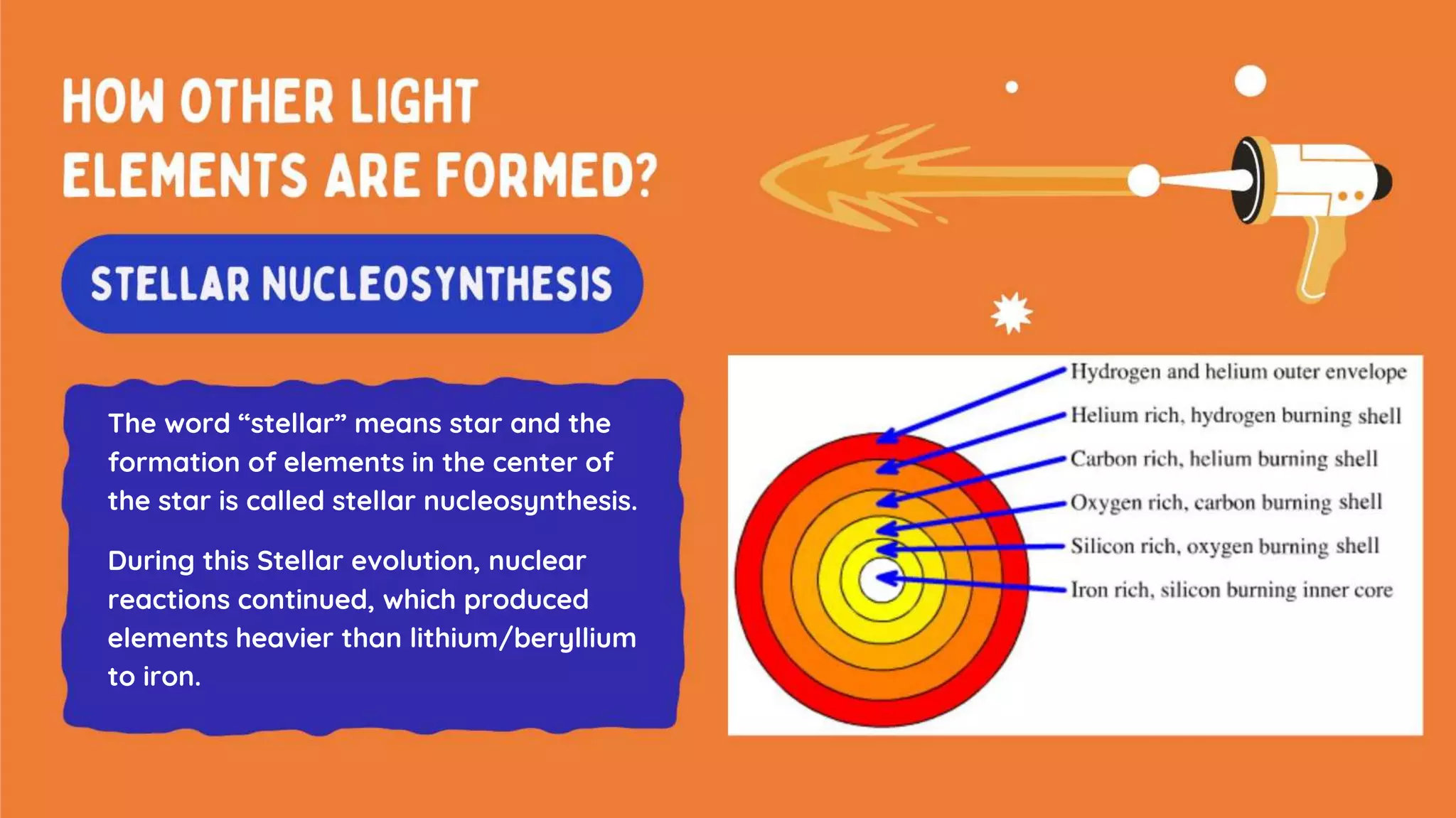
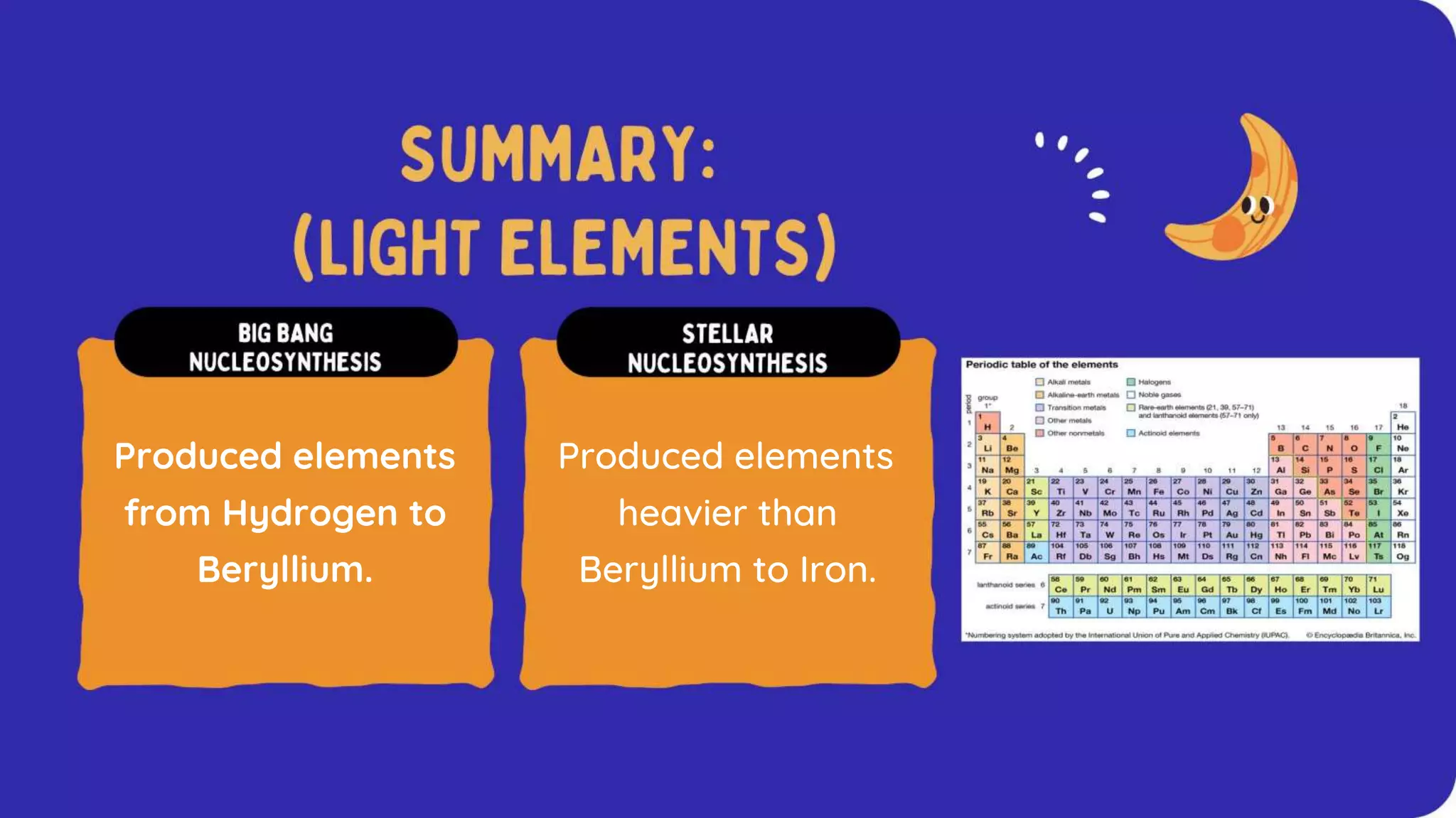
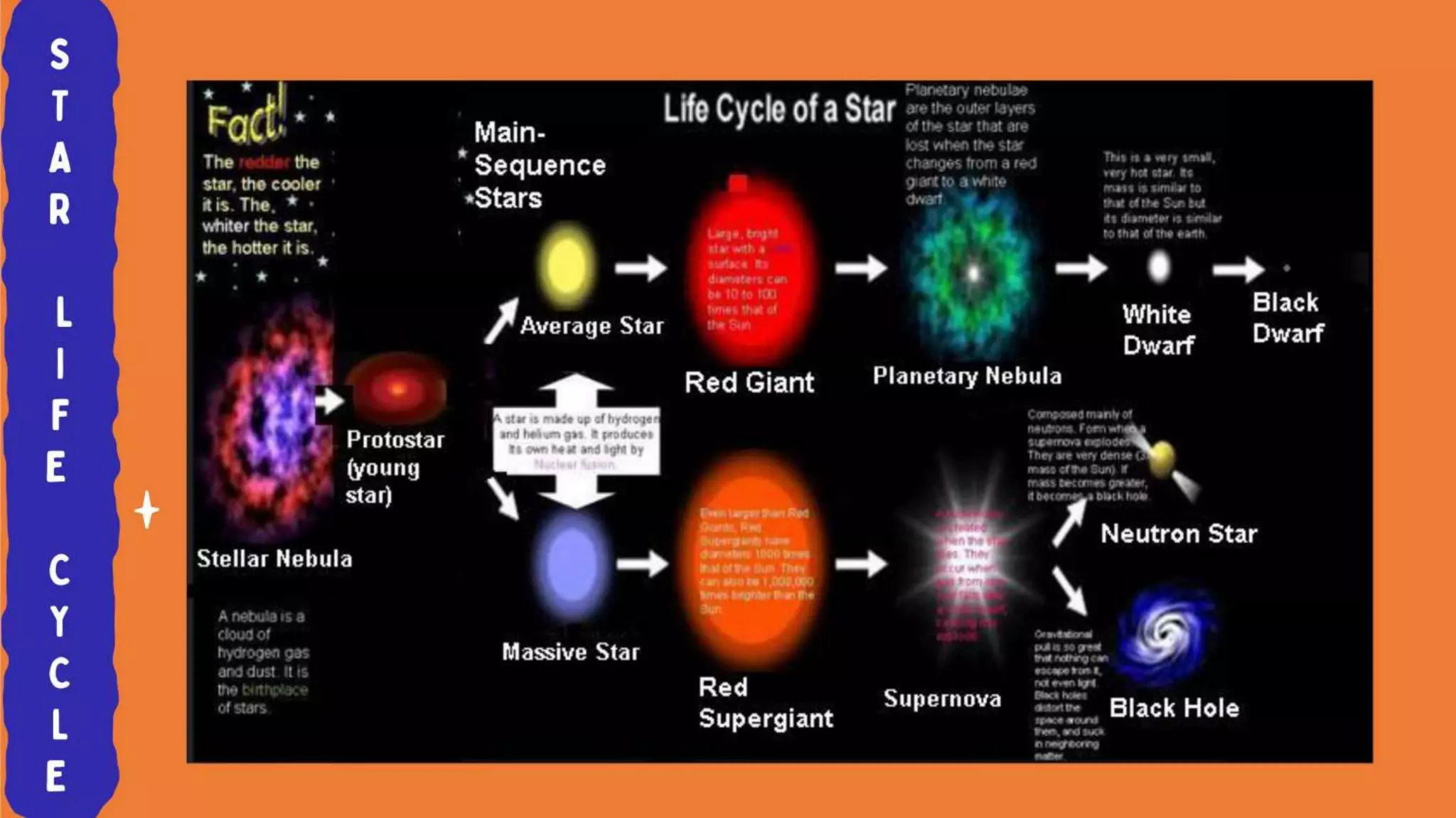
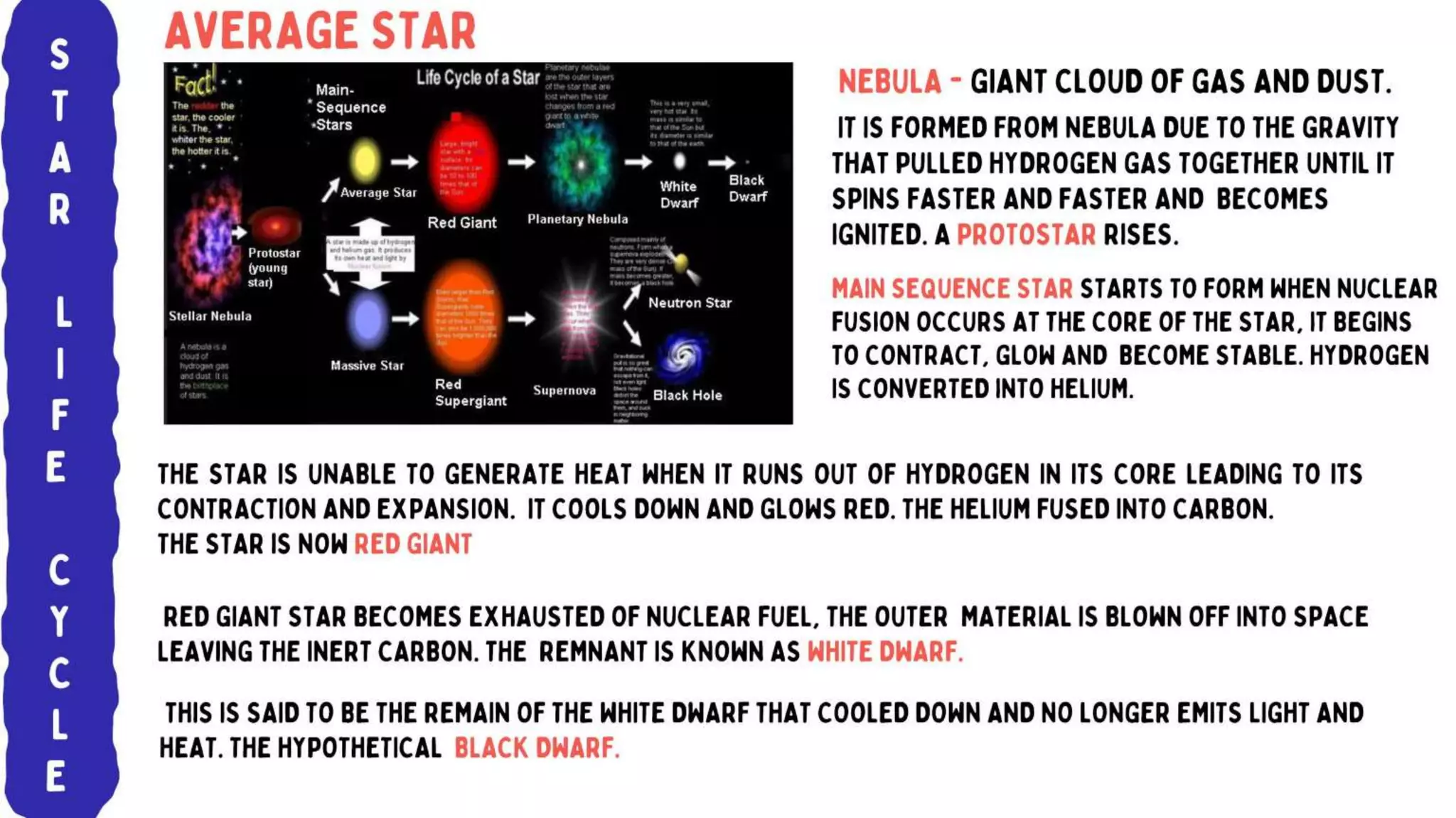
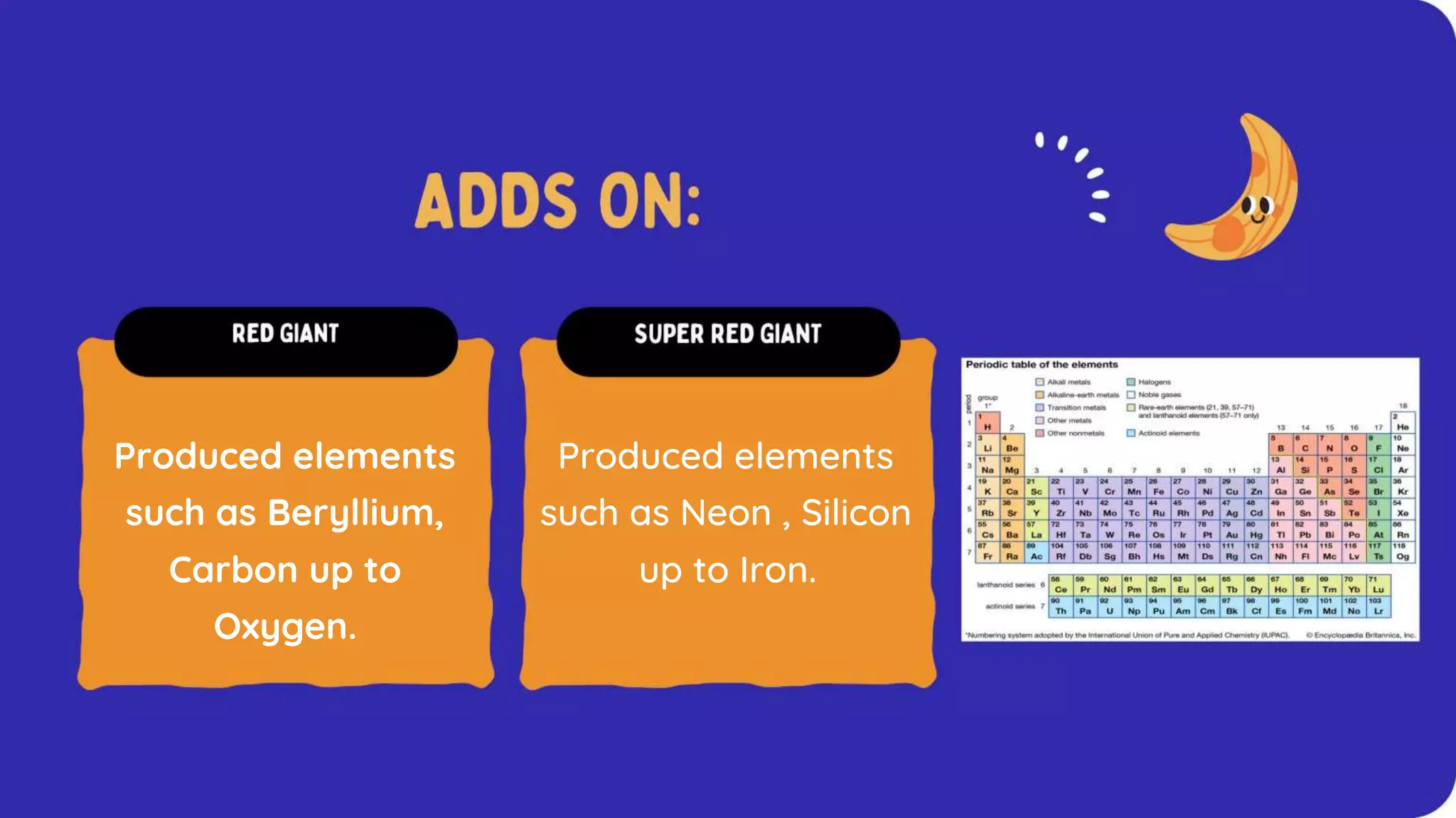
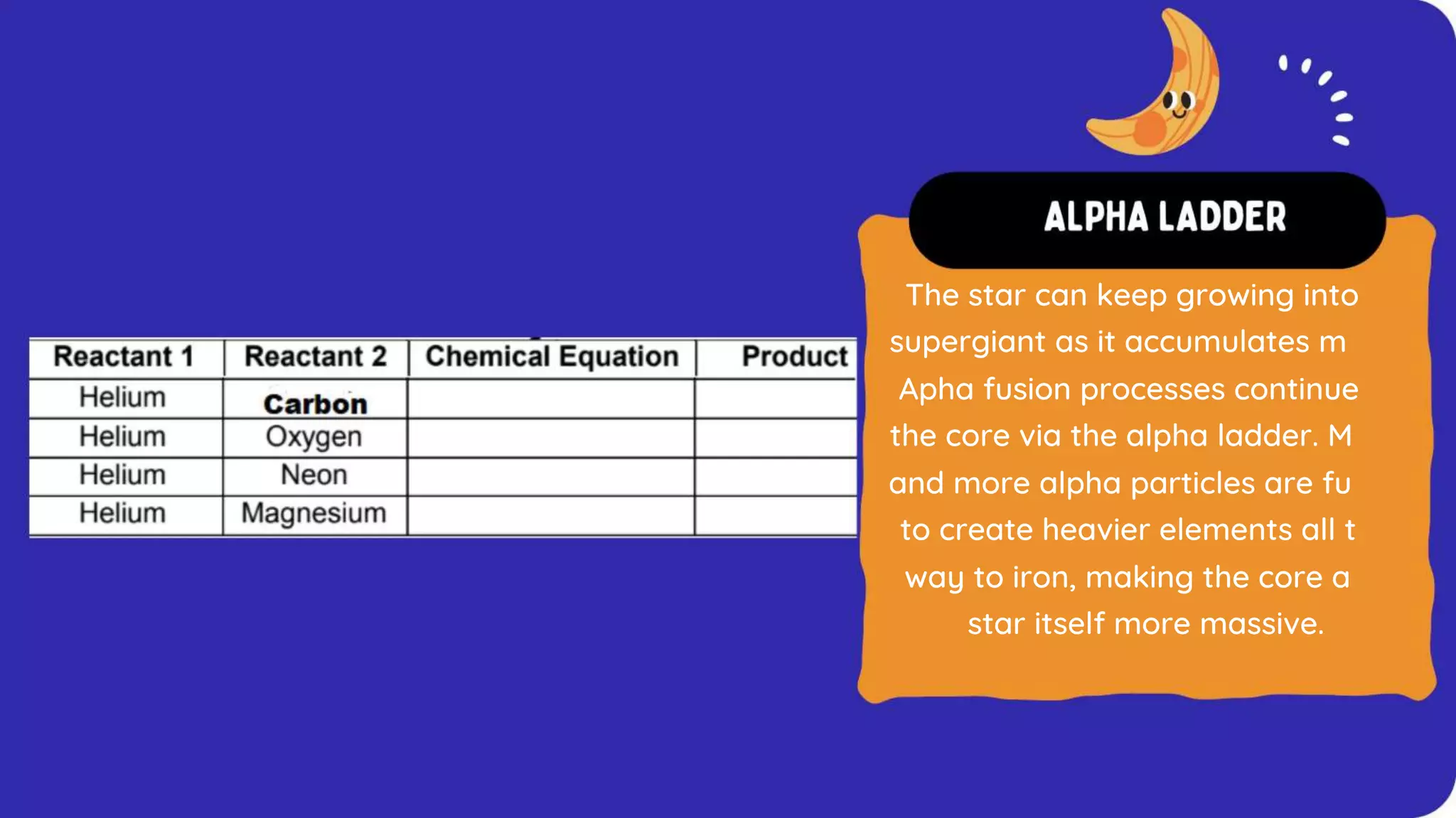
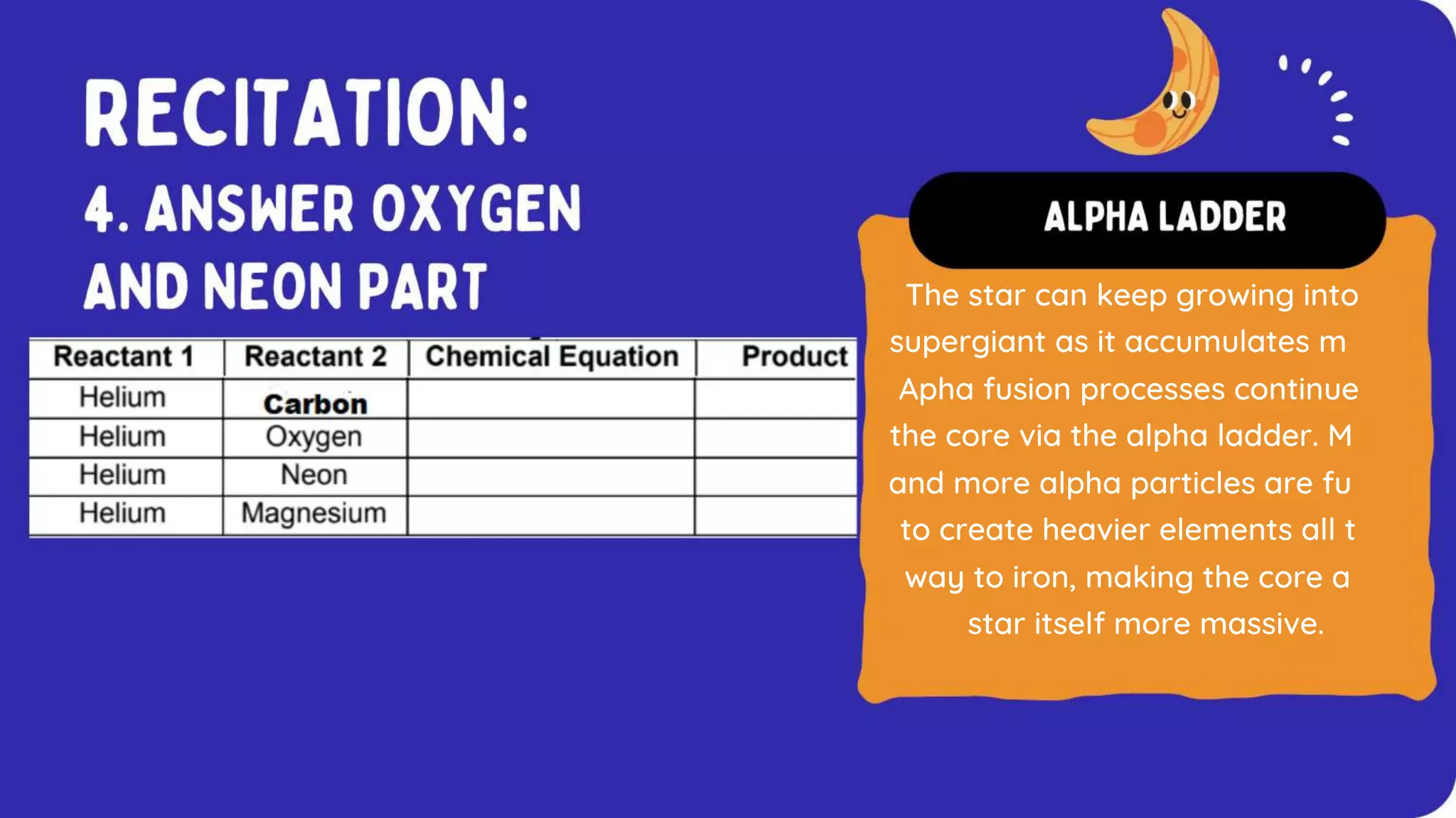
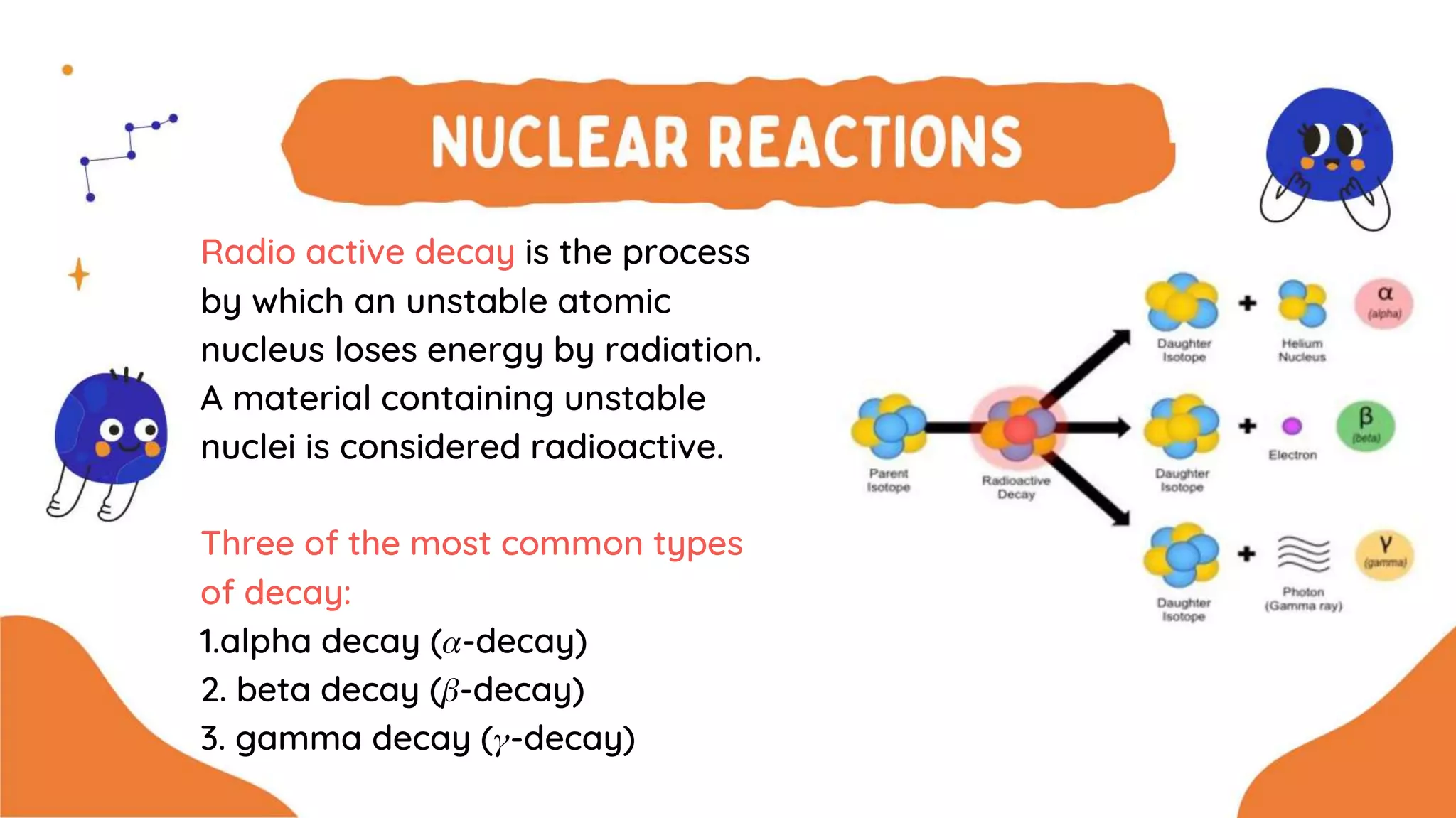
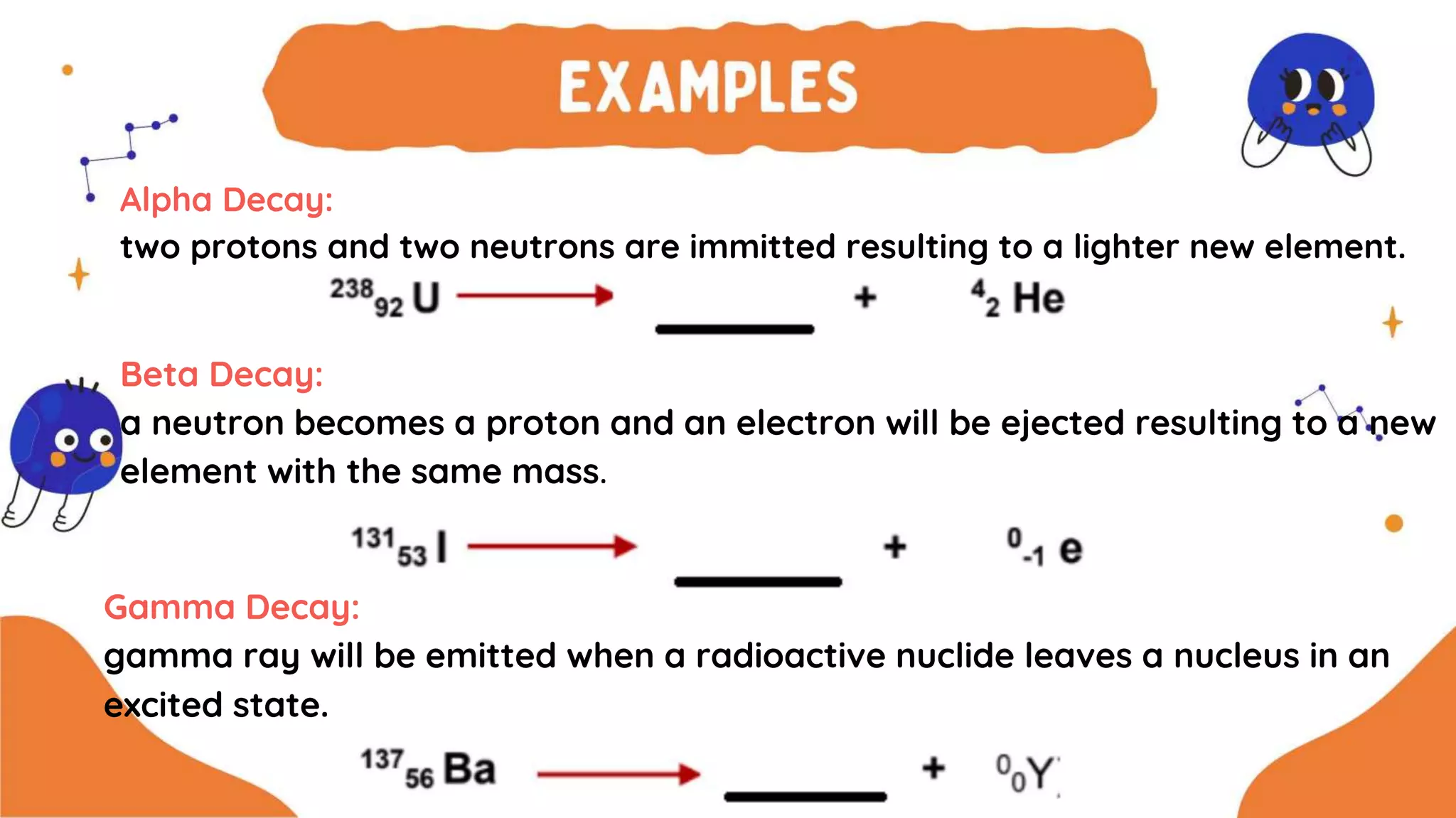
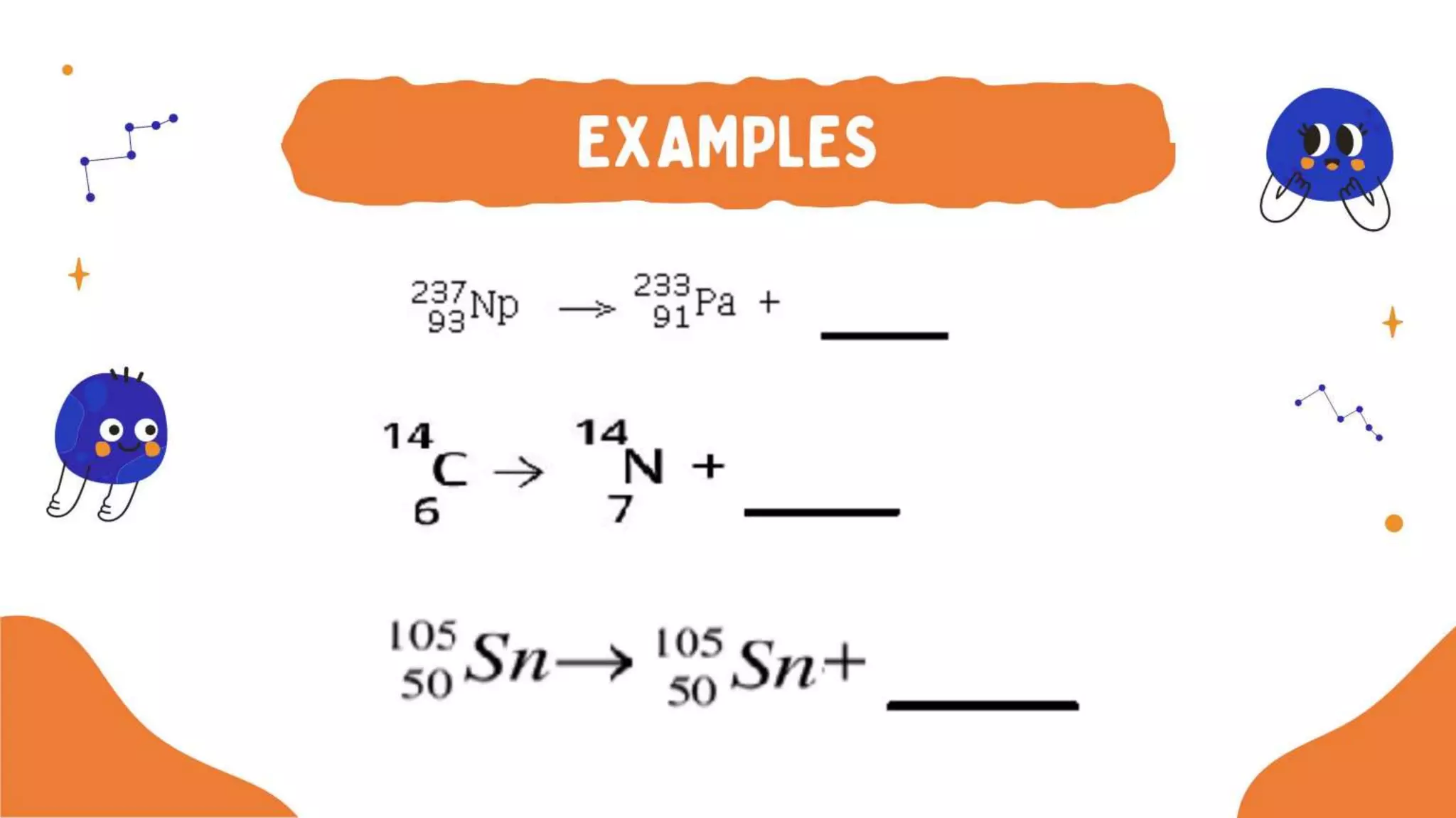
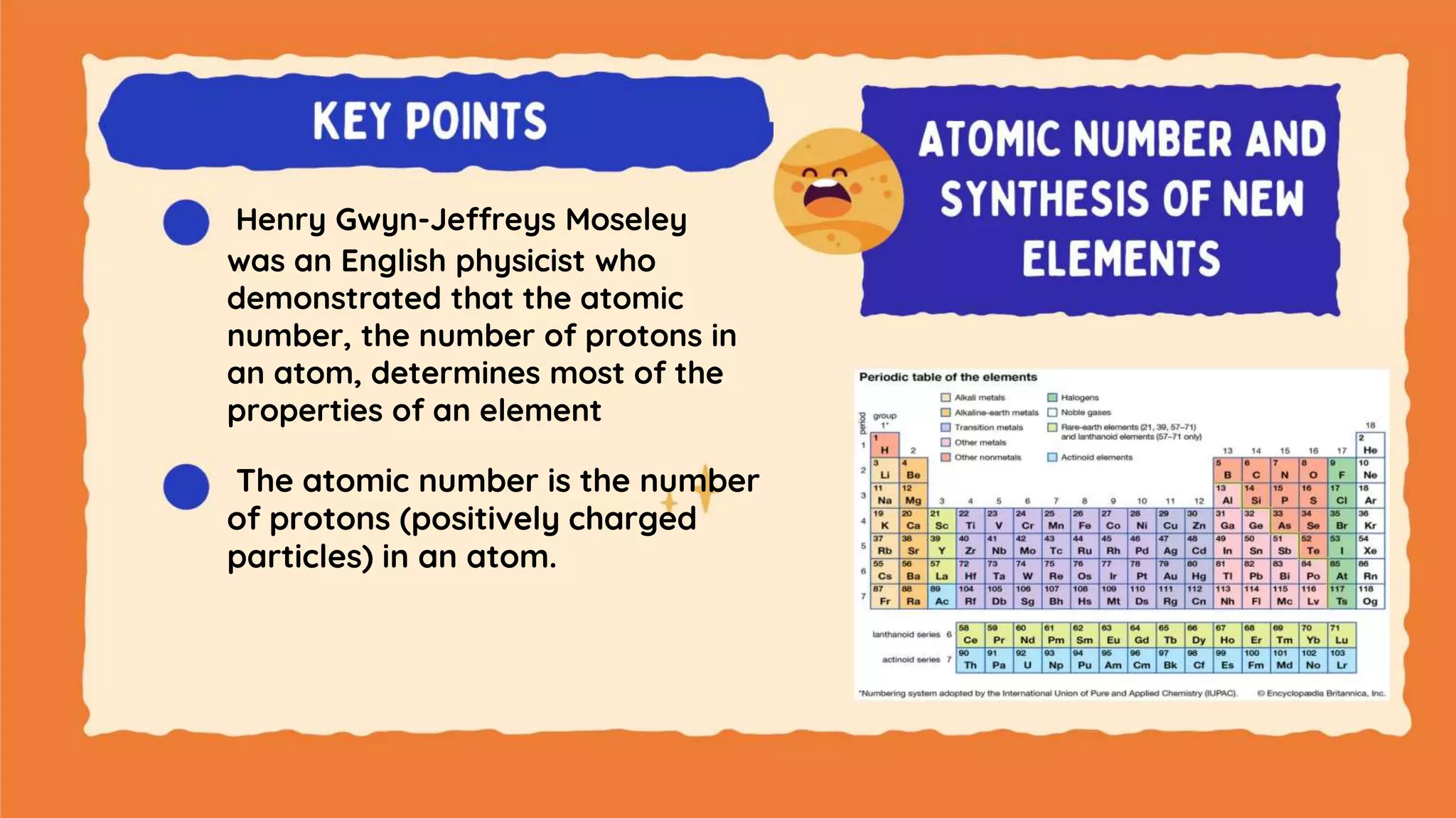
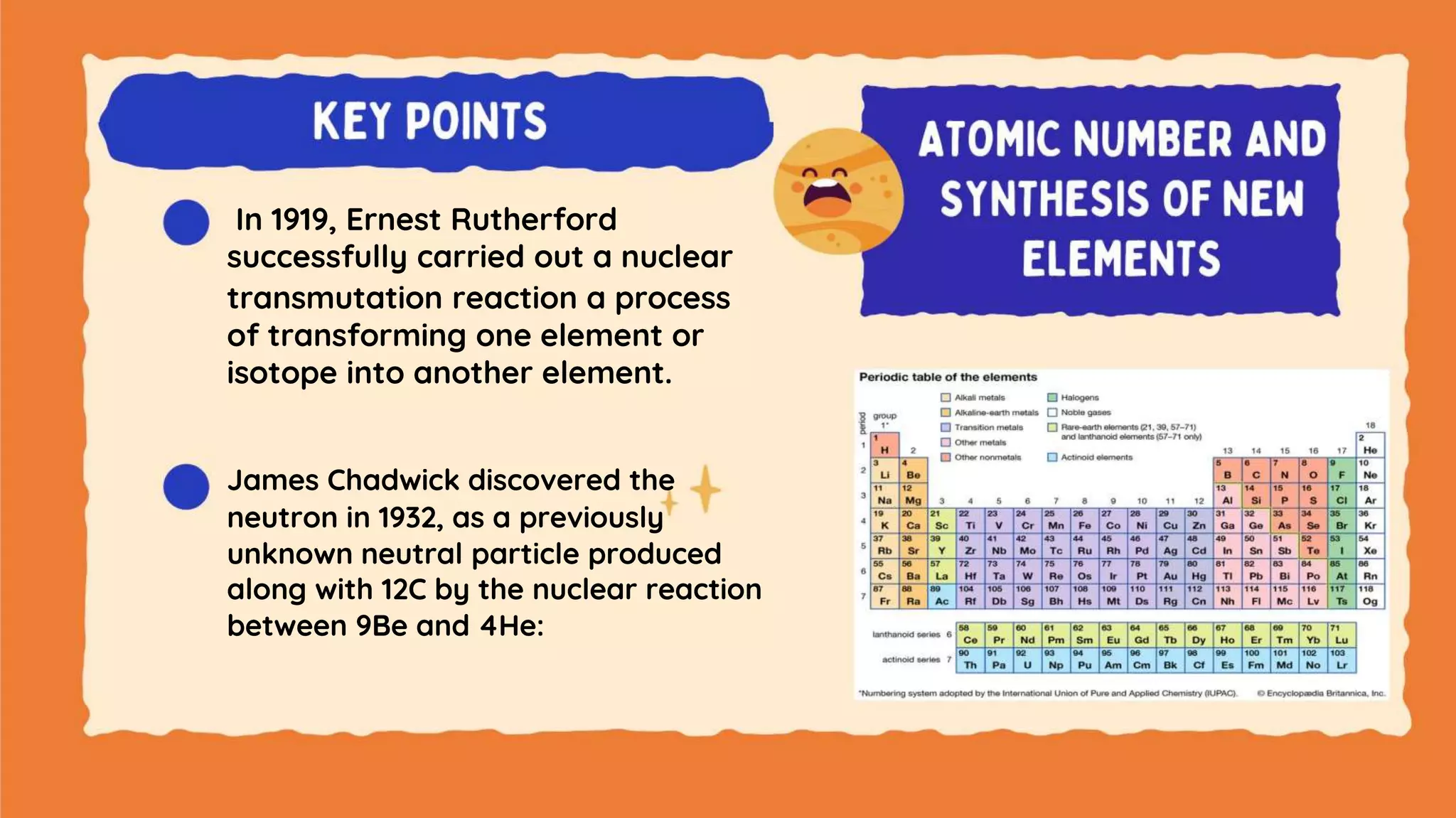
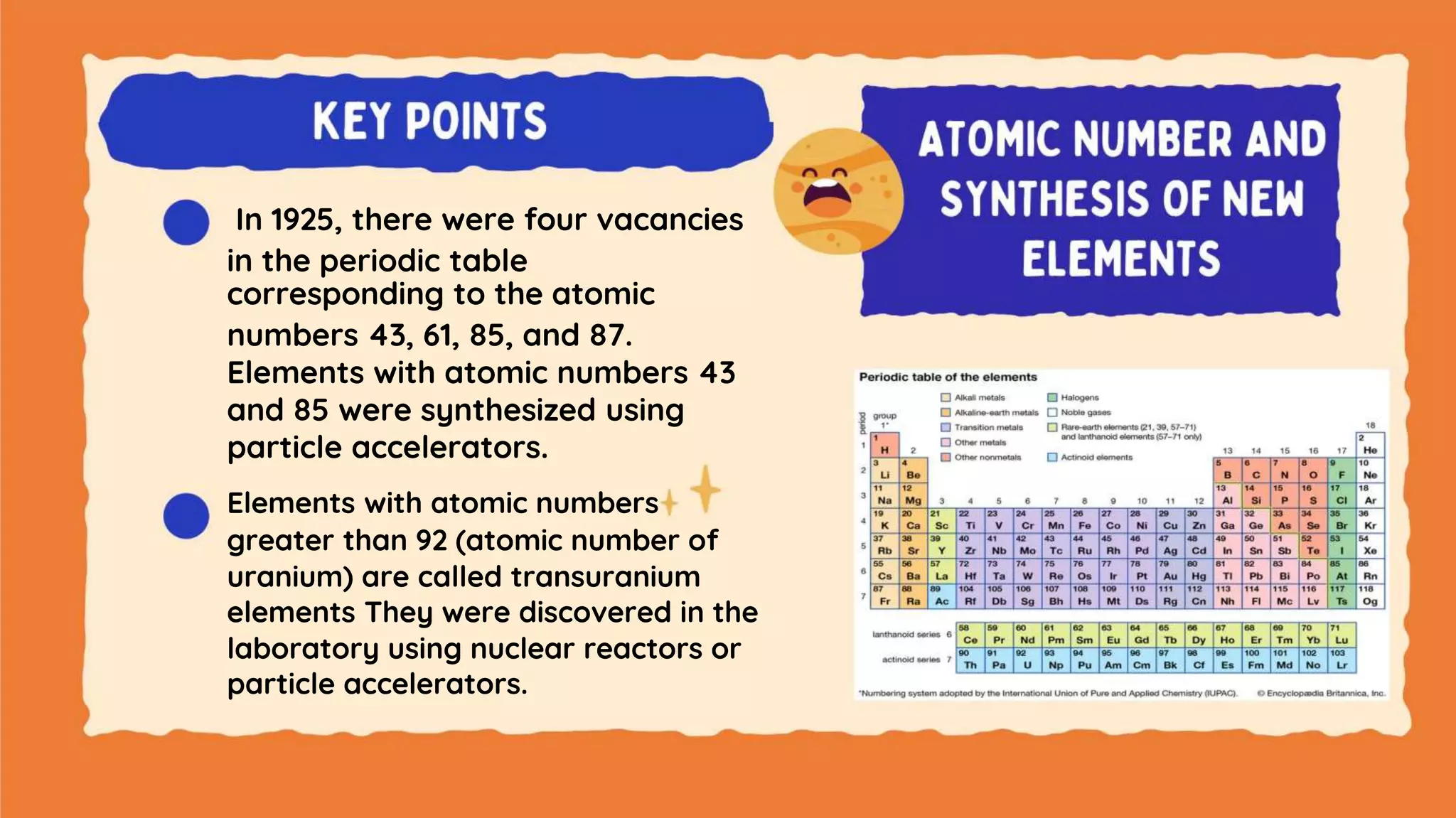
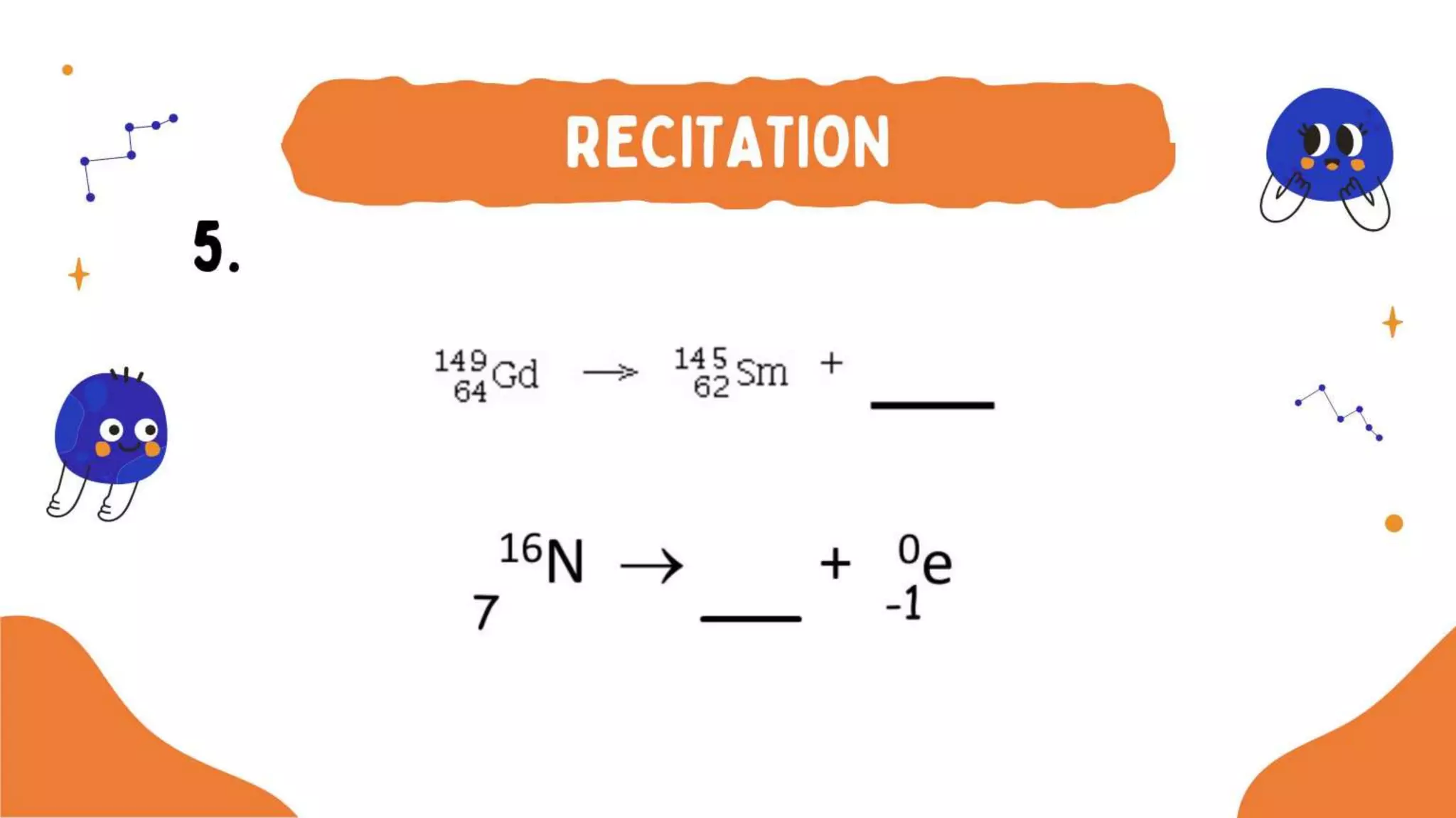The document discusses the formation of elements during stellar formation and evolution. According to the Big Bang theory, the early universe contained only light elements like hydrogen, helium, and lithium. During stellar nucleosynthesis in the centers of stars, nuclear fusion reactions produced heavier elements up to iron. Later stellar evolution and supernova explosions produced even heavier elements. Nuclear reactions and radioactive decay were later used in laboratories to synthesize new elements and fill in gaps in the periodic table.