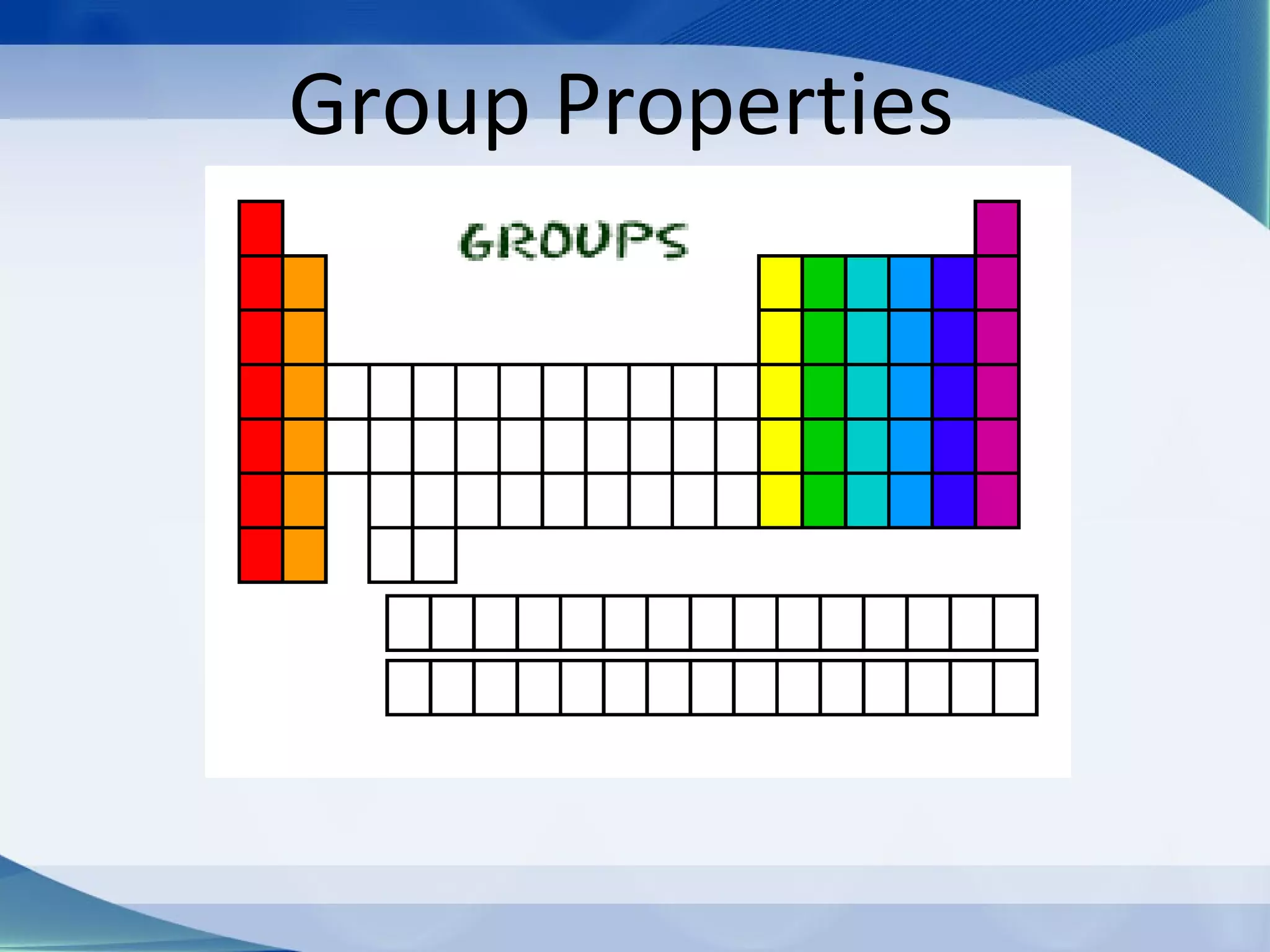
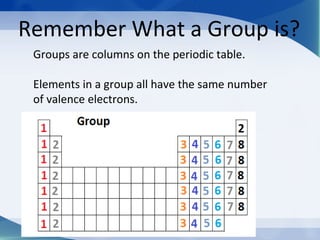
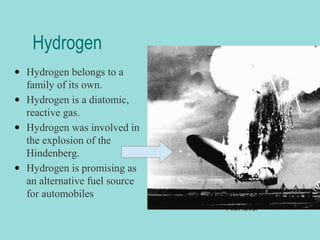
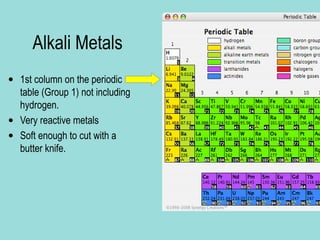
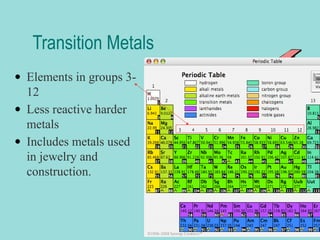
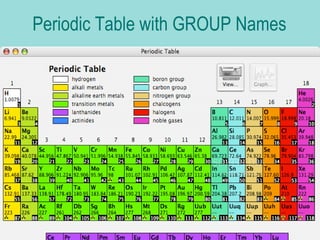
Groups are columns on the periodic table that contain elements with the same number of valence electrons. The document discusses several important groups including hydrogen, alkali metals, alkaline earth metals, transition metals, boron family, carbon family, nitrogen family, oxygen family, halogens, and noble gases. It provides key details about the location of each group on the periodic table and chemical properties common to the elements within each group.