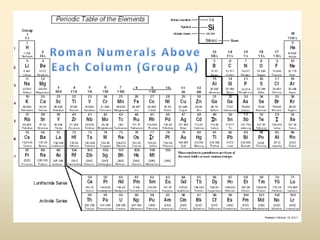
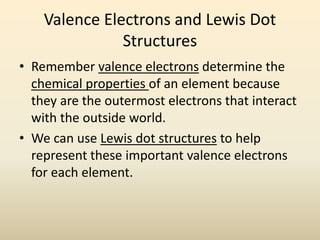
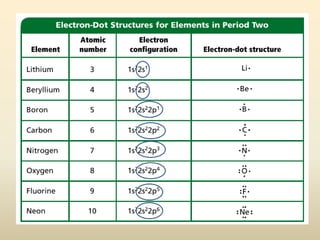
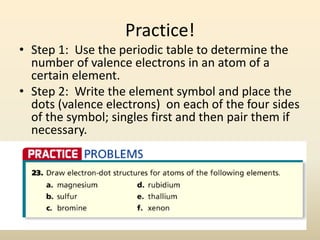
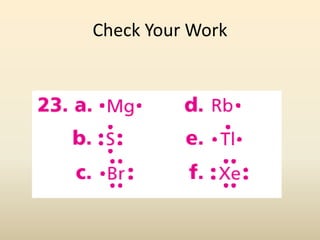
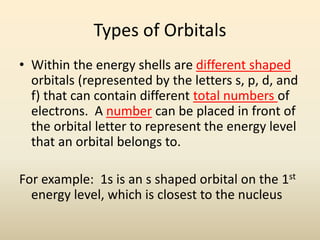
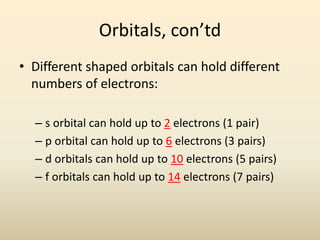
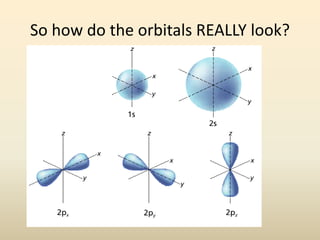
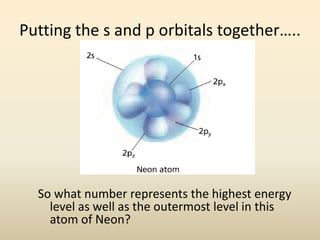
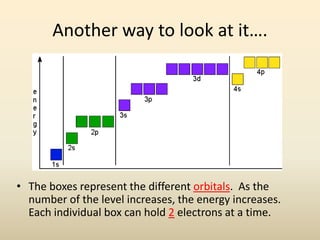
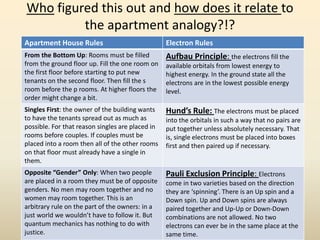
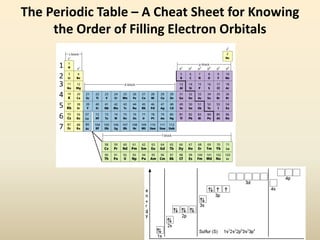
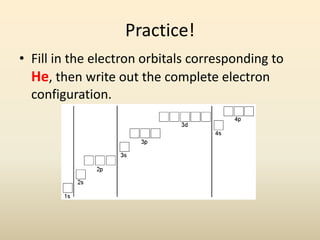
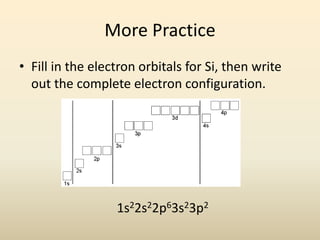
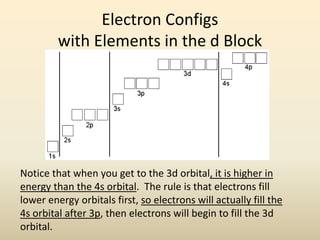
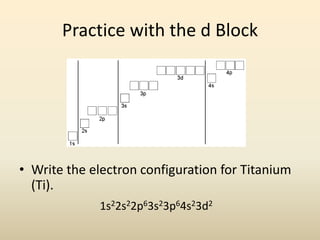
Electron configurations describe the arrangement of electrons in an atom. Electrons occupy different energy levels and orbitals within these levels. The Aufbau principle states that electrons fill the lowest available energy levels first from the s, p, d, and f orbitals. Hund's rule specifies that electrons occupy different orbitals within the same energy level before pairing up. The Pauli exclusion principle indicates that no two electrons can occupy the same orbital. Noble gas notation provides a shorthand for writing electron configurations.

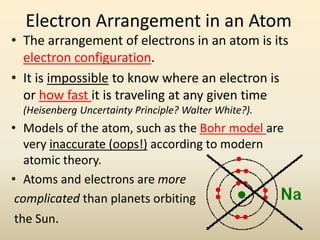



![Noble Gas Notation
• This is much shorter and more convenient than writing
out the entire electron configuration.
• Use the symbol for the noble gas that is just before the
element you are configuring. (The noble gas and the
element will have the same configuration, or inner
electron structure, up to that point)
• Then complete the configuration that comes after the
noble gas for the element in question.
• Example: The complete configuration for Na is
1s22s22p63s1. Neon is the noble gas that comes before
Na on the periodic table. So the noble gas notation for
Na would be: [Ne]3s1](https://image.slidesharecdn.com/electron-configurations-powerpoint-230620151020-3d033406/85/Electron-Configurations-powerpoint-pdf-19-320.jpg)
![Practice!
• Write the noble gas notation for the following
elements:
– Chlorine
– Beryllium
[Ne]3s23p5
[He]2s2](https://image.slidesharecdn.com/electron-configurations-powerpoint-230620151020-3d033406/85/Electron-Configurations-powerpoint-pdf-20-320.jpg)
![More Practice
• Which element has the following
configuration: [Xe]6s2?
Barium](https://image.slidesharecdn.com/electron-configurations-powerpoint-230620151020-3d033406/85/Electron-Configurations-powerpoint-pdf-21-320.jpg)