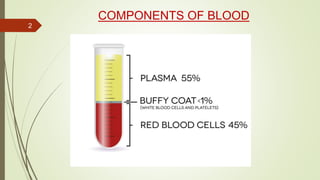
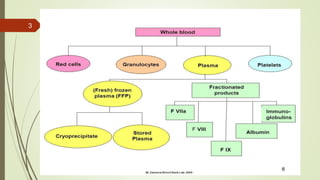
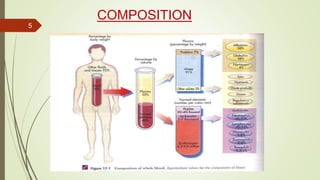
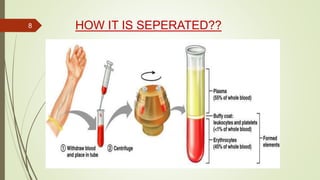
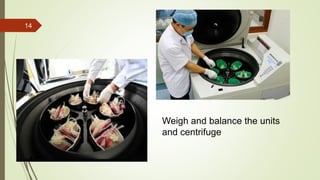
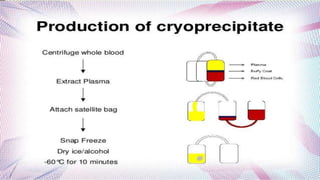
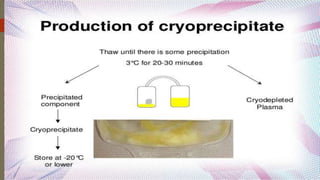
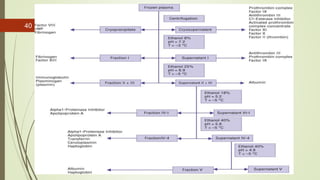
This document provides information on the various components that can be derived from human plasma. It begins by explaining that plasma is the straw-colored liquid portion of blood that suspends the blood cells and contains water, salts, enzymes, antibodies and proteins. The document then describes different plasma derivatives like fresh frozen plasma, cryoprecipitate, coagulation factors, albumin and fibrin glue/sealants. It provides details on the preparation, composition, storage, indications and administration for each plasma component.