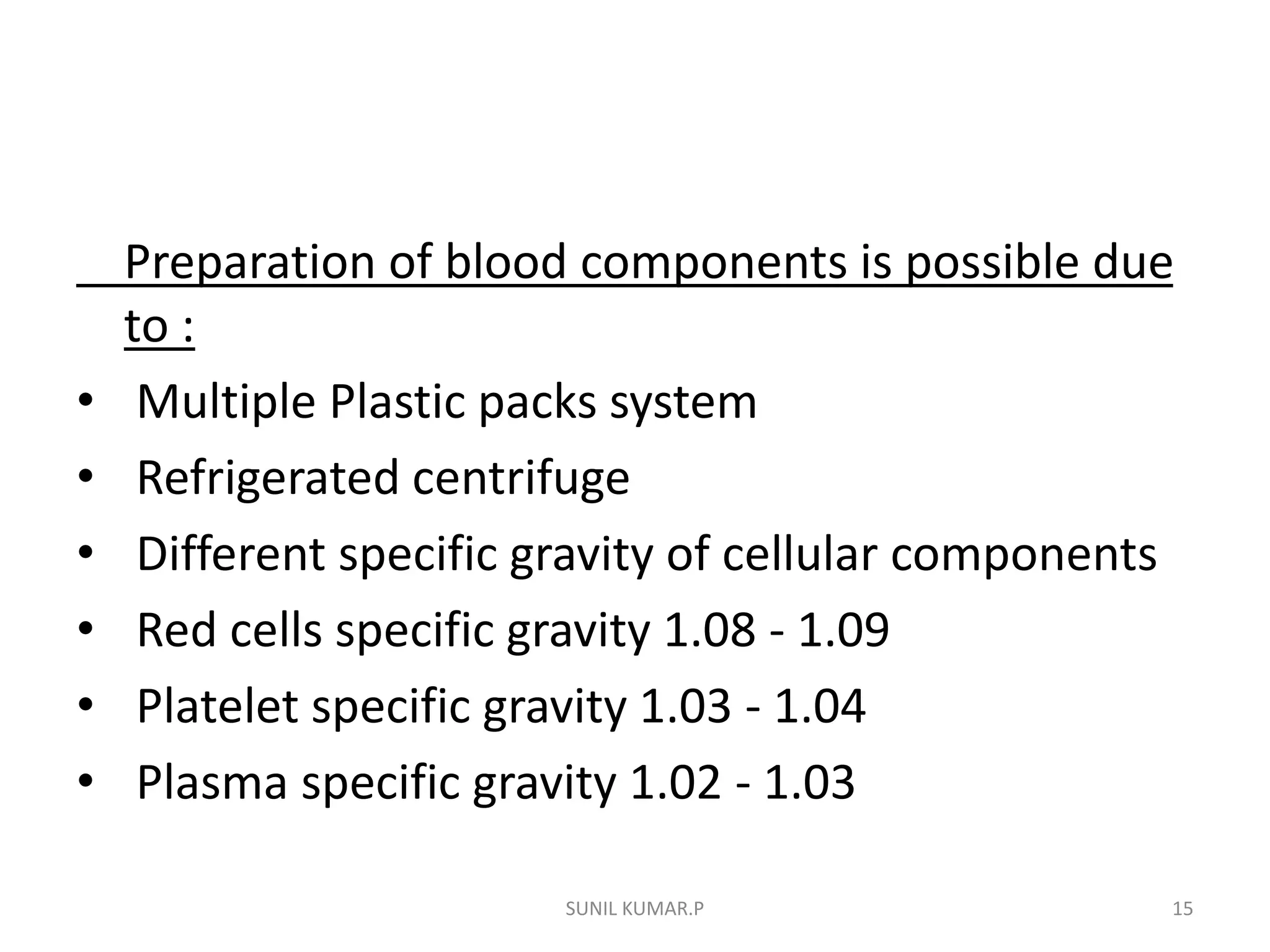
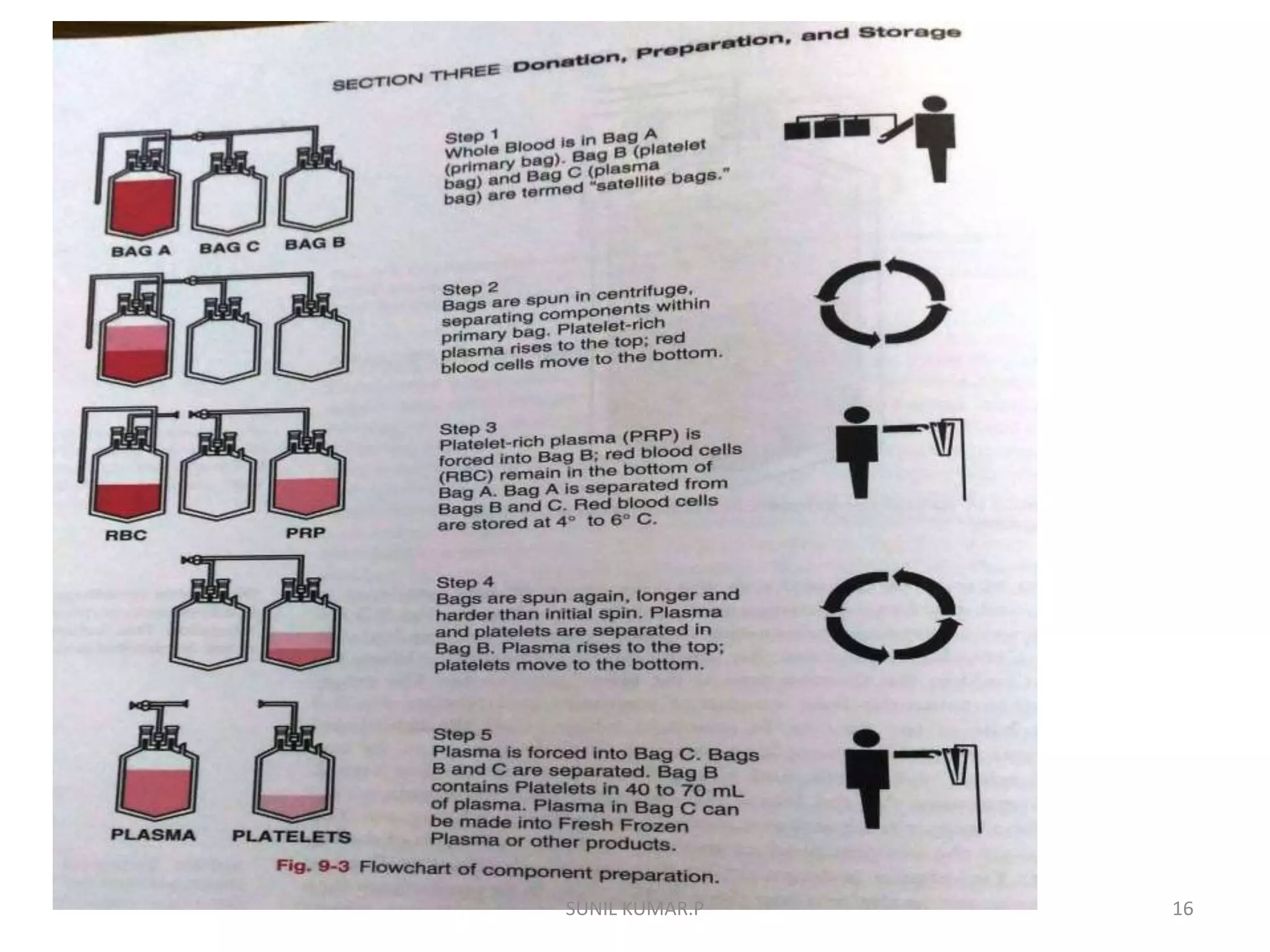
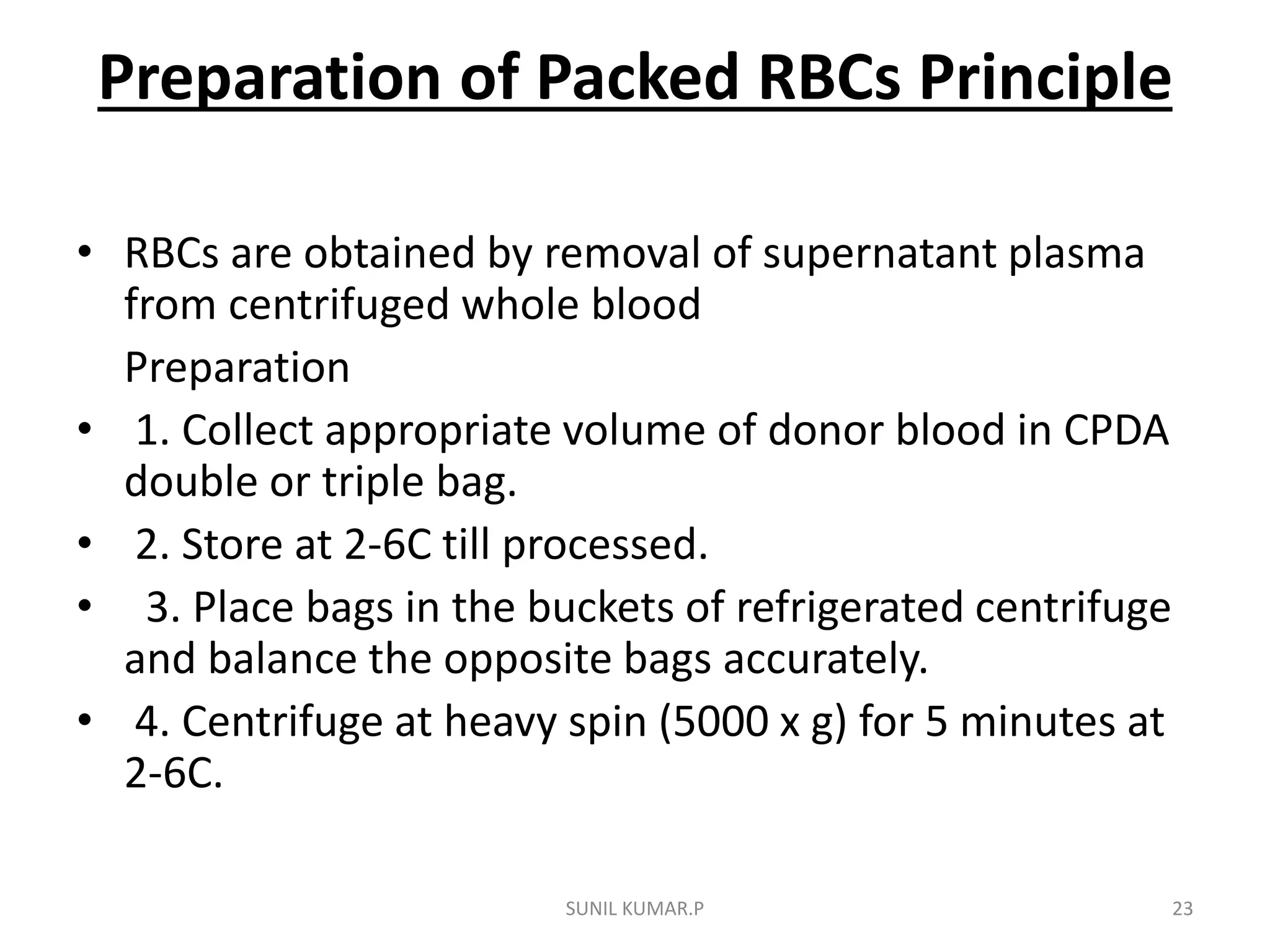
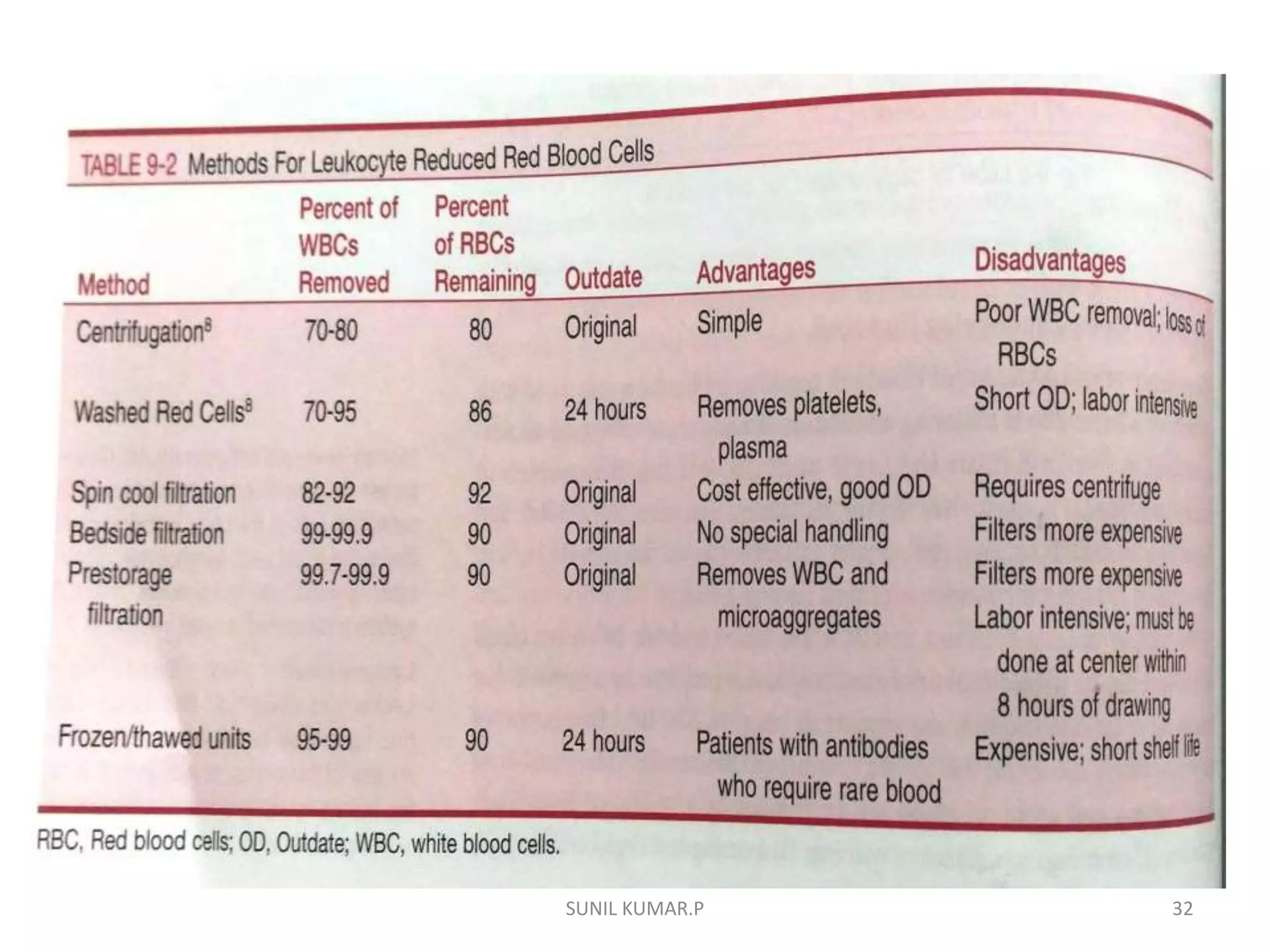
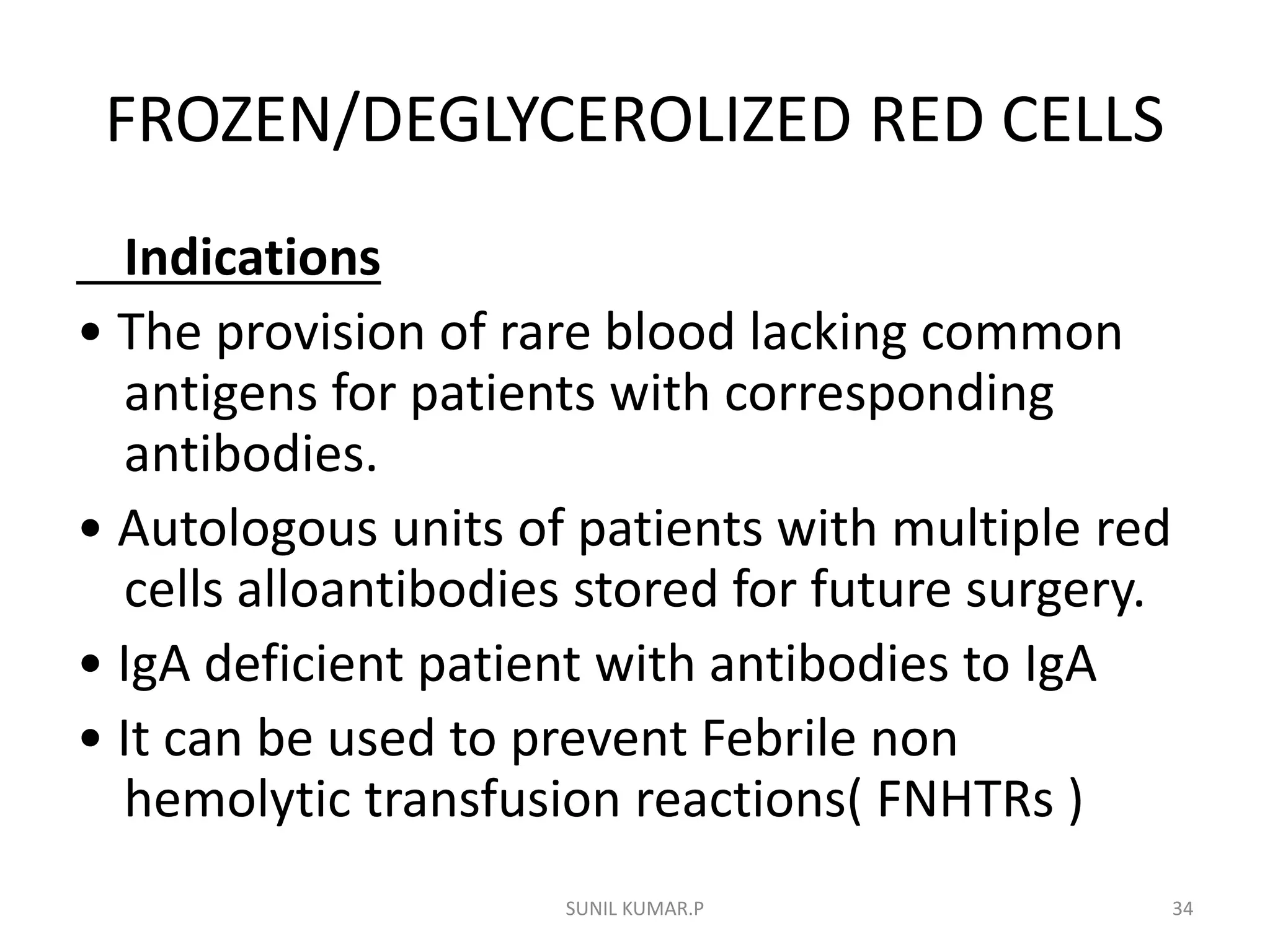
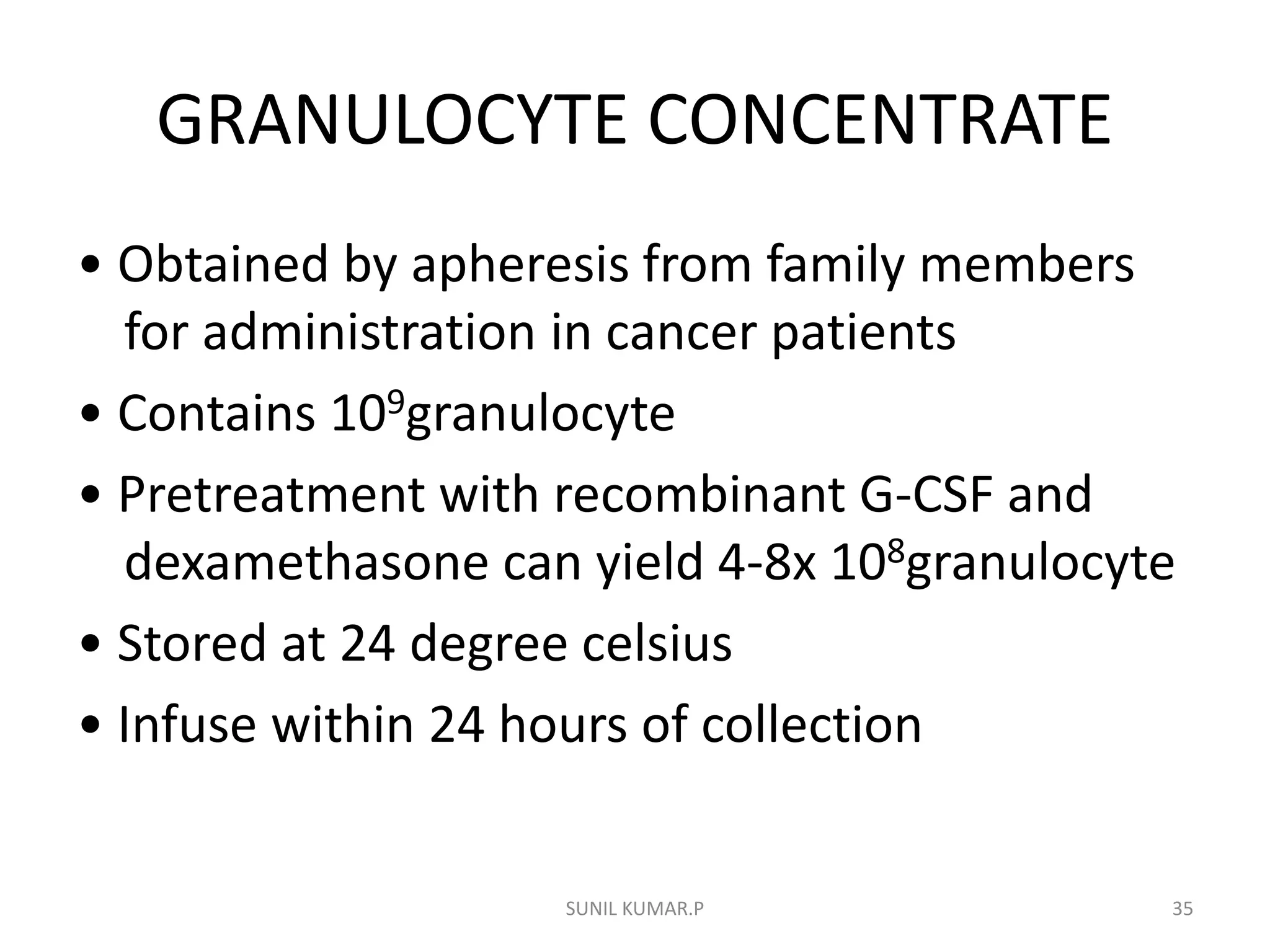
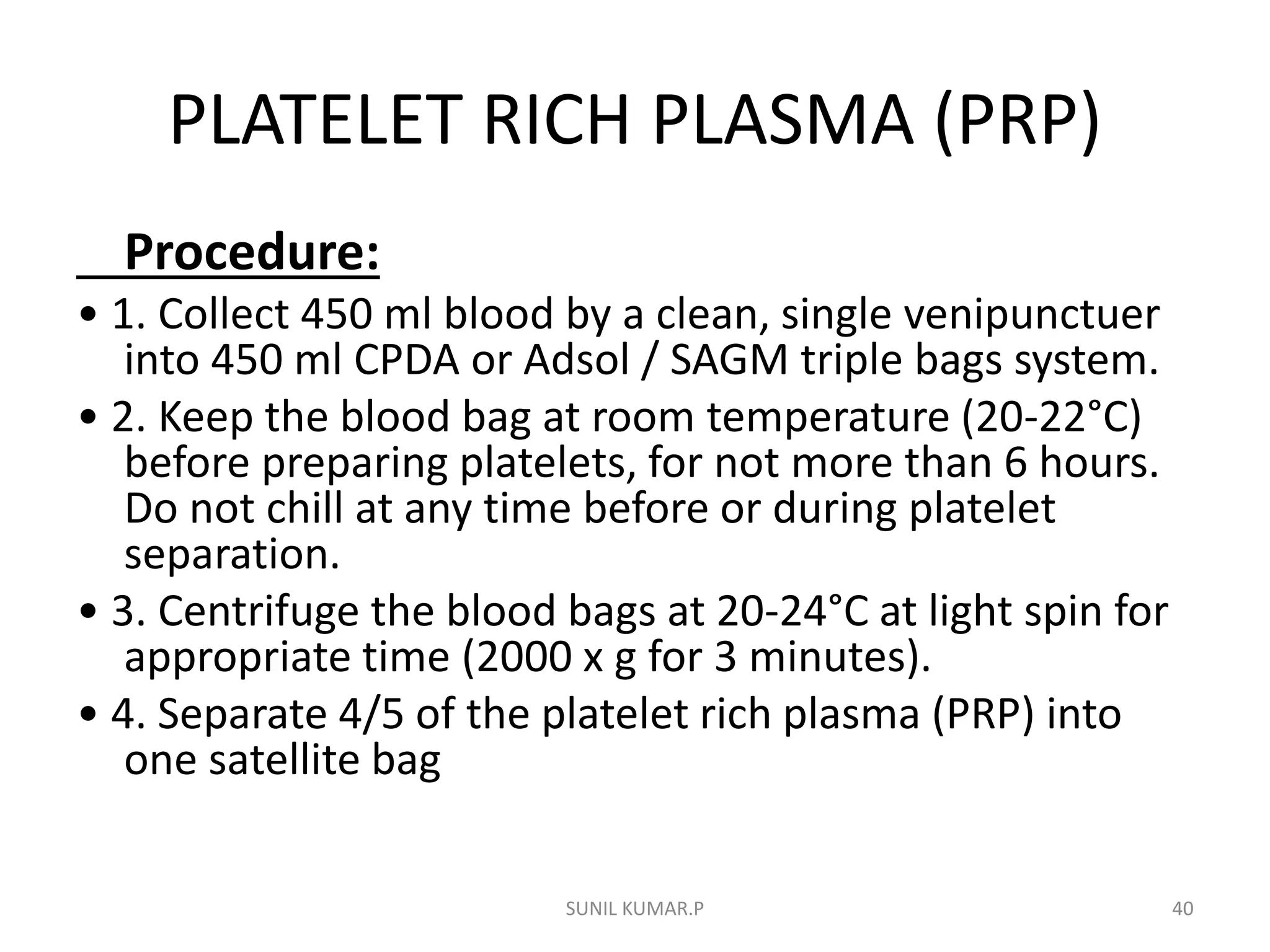
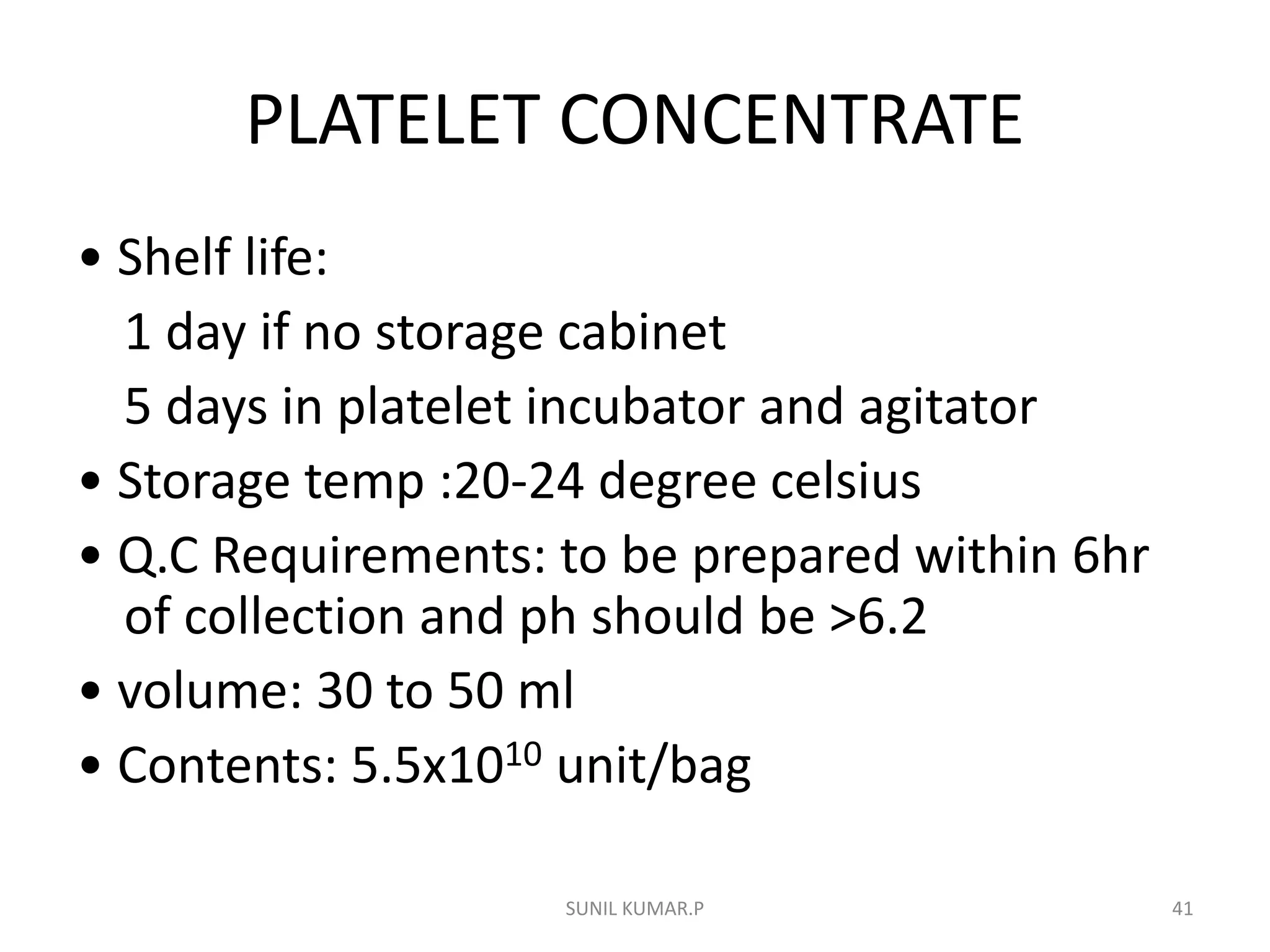
Blood can be separated into components to meet most transfusion needs while minimizing risks. Effective separation requires centrifugation based on differences in specific gravity of components. Common components prepared include packed red blood cells, platelet concentrates, fresh frozen plasma, and granulocyte concentrates. Preparation of blood components allows for optimal use of donated blood by providing only the required constituent to the patient in need.