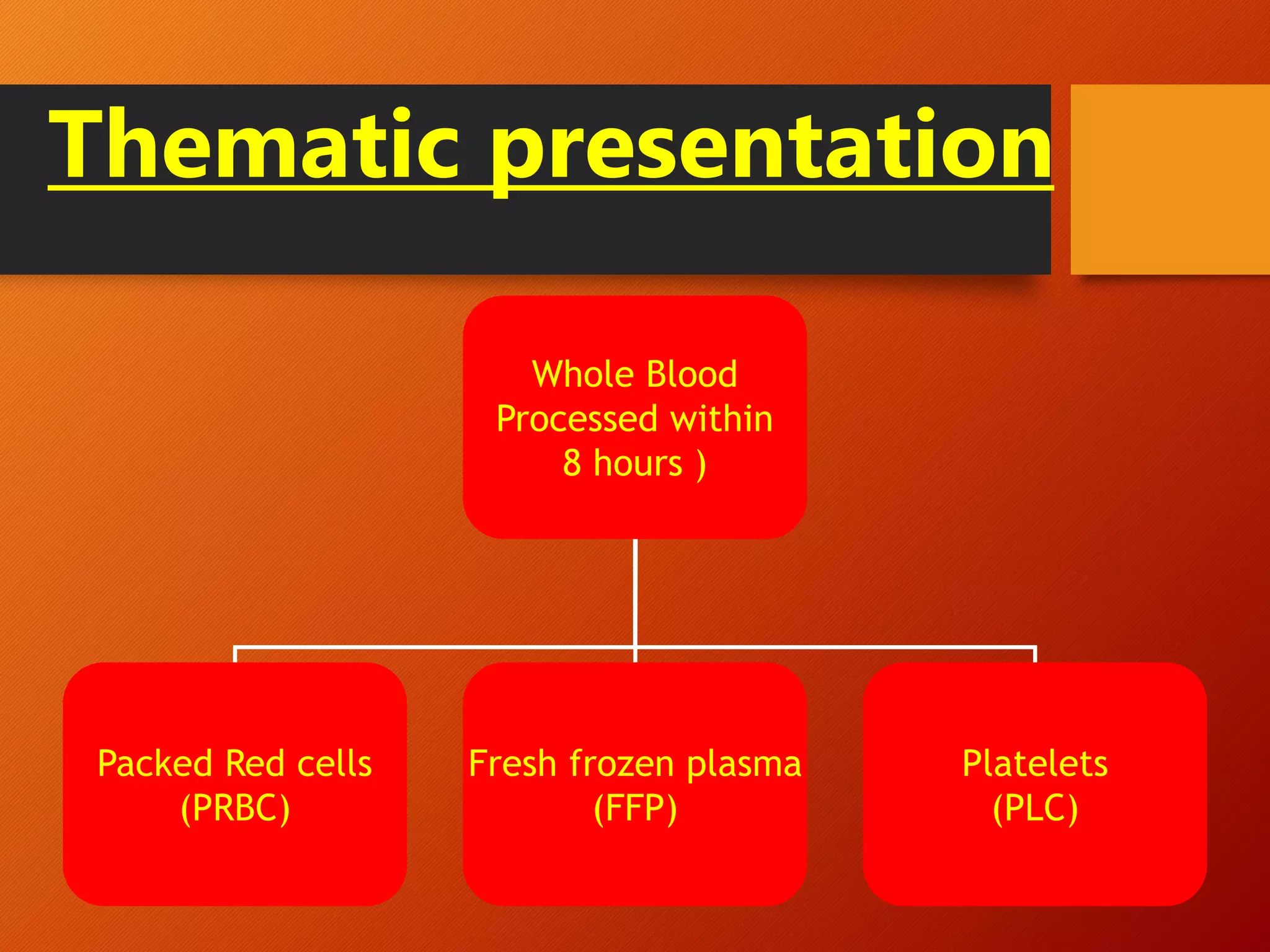
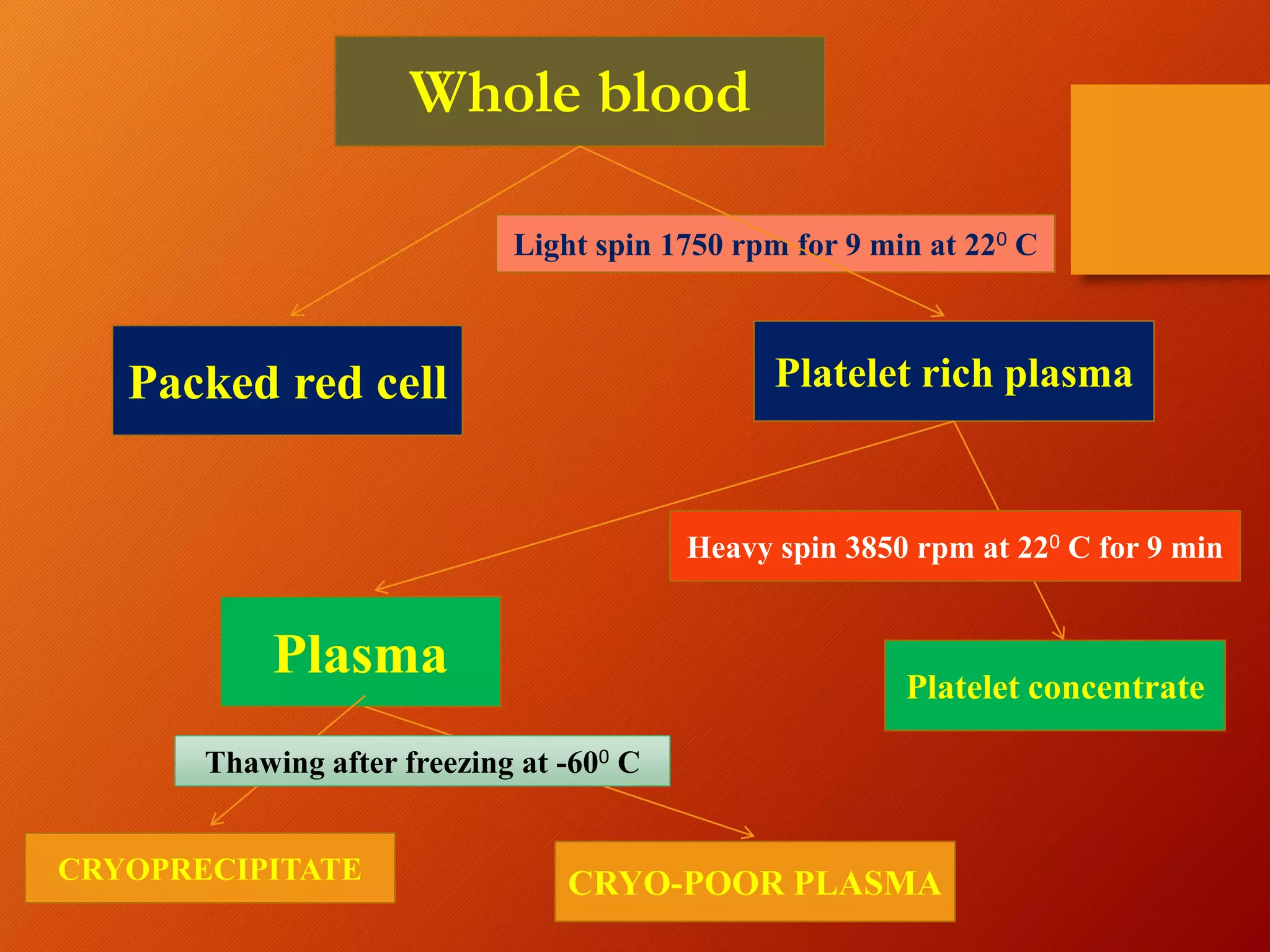
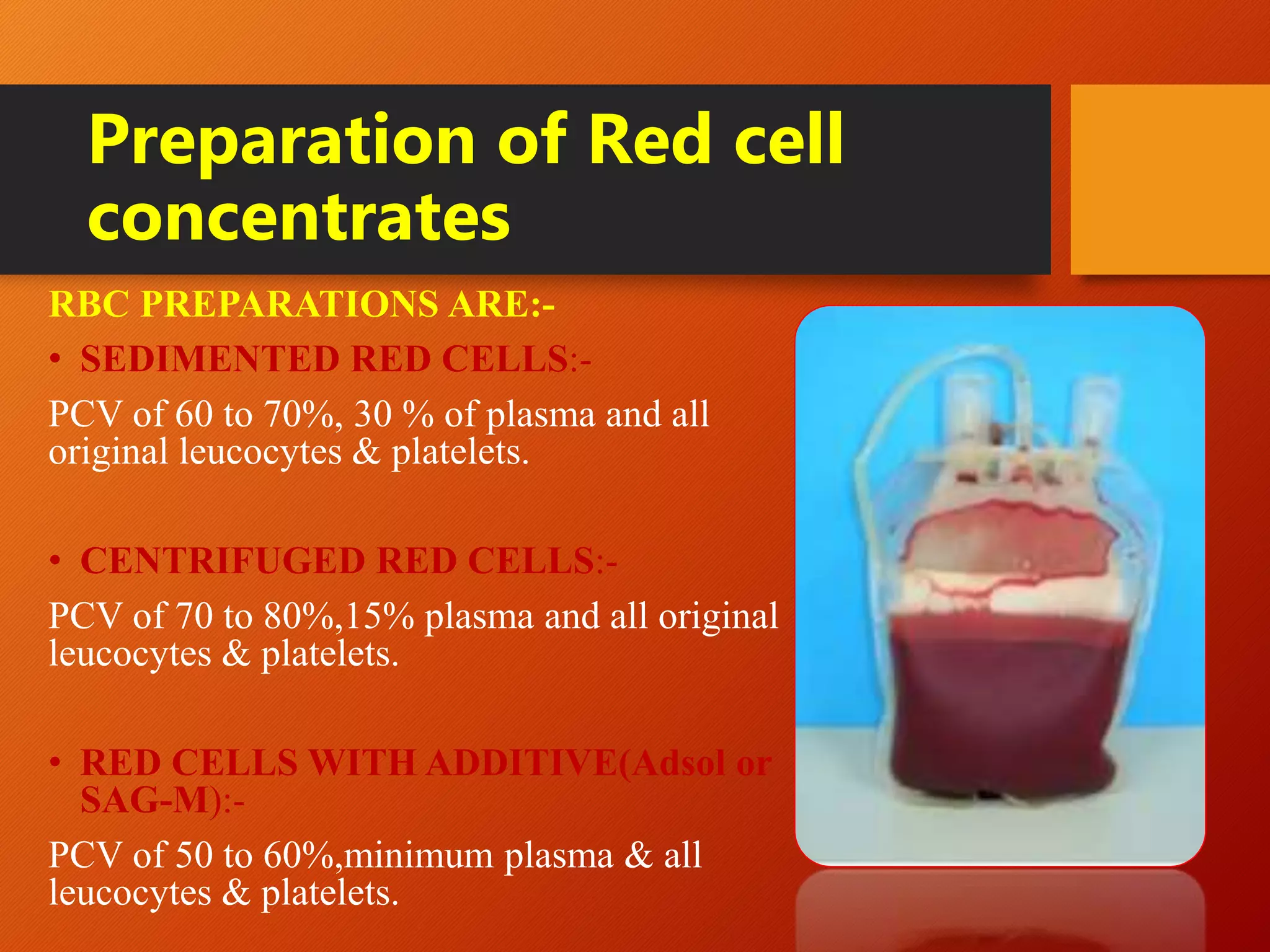
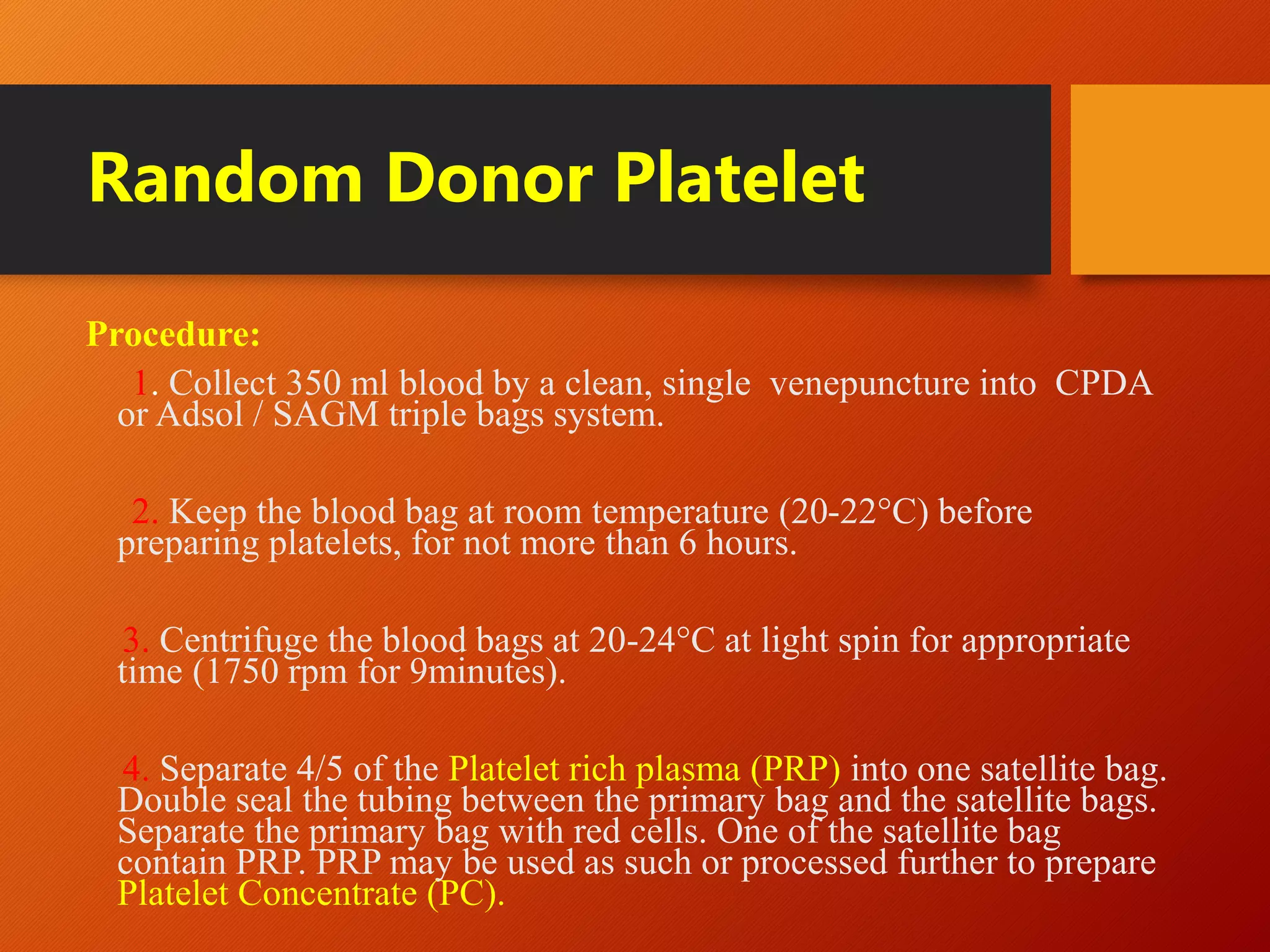
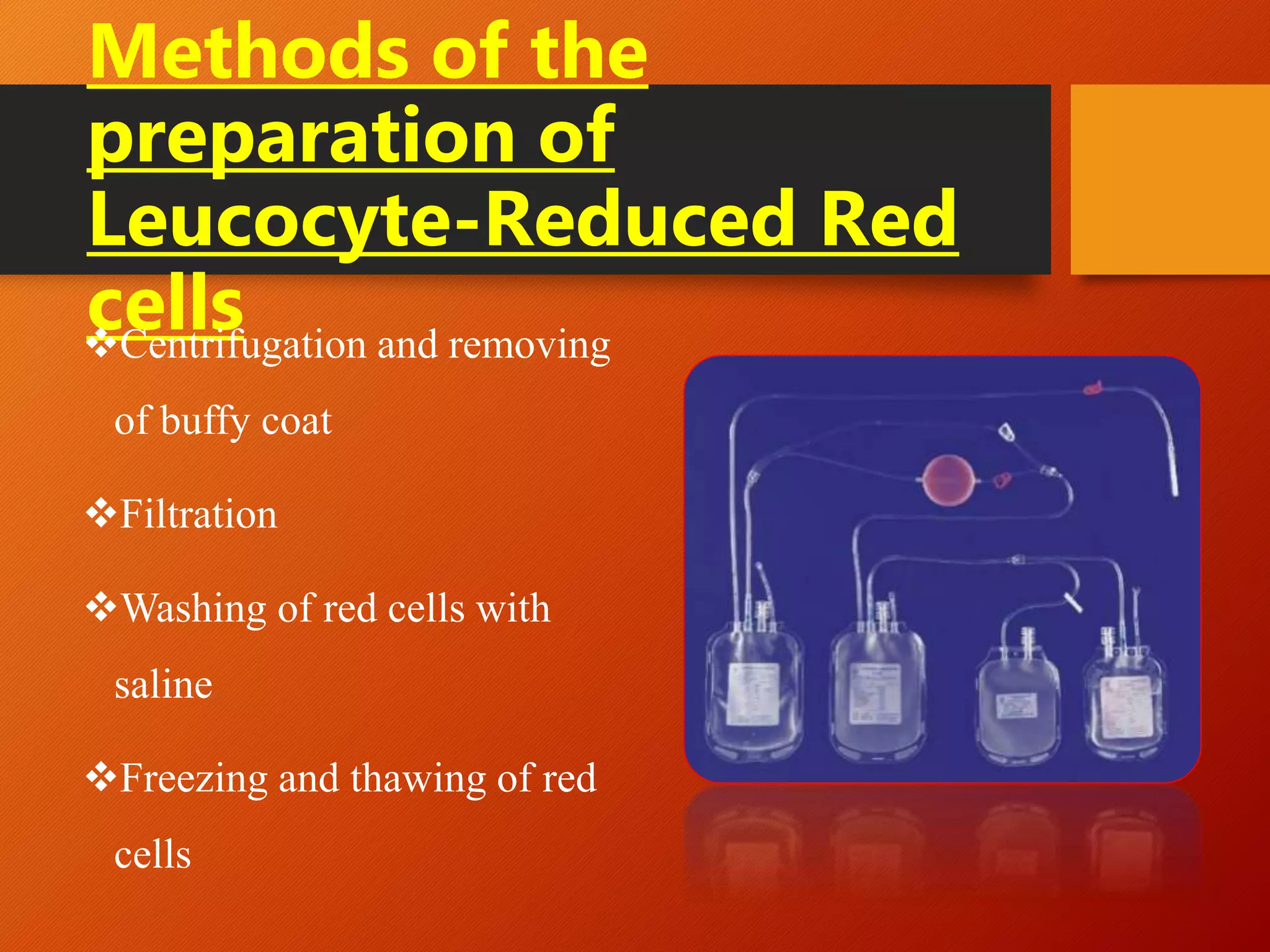
This document summarizes the principles and history of blood component separation and the indications for various blood components. It discusses how separating blood into components allows for more targeted transfusions based on patient needs. The key components discussed are red blood cells, platelets, fresh frozen plasma, and cryoprecipitate. The document outlines the processes for preparing these components, including centrifugation techniques, and their various clinical uses. It emphasizes that component therapy maximizes blood resources and allows multiple patients to be treated from a single blood donation.







![Mathematical formulae
RPM Formula:
RPM = √[RCF/(r x 1.118)] x 1 x 105
RCF (g Force) = Relative Centrifugal Force
r = radius of centrifuge rotor in cm
(rotational radius)
G Force (RCF) Formula:
Relative Centrifugal Force (RCF) in g = (RPM)2 x 1.118 x 10-5 x r
r = radius of centrifuge rotor in cm
(rotational radius)](https://image.slidesharecdn.com/bloodcomponentprinciplesofseparationindication-221214142019-49f52c32/75/Blood-component-Principles-of-separation-indication-pptx-10-2048.jpg)