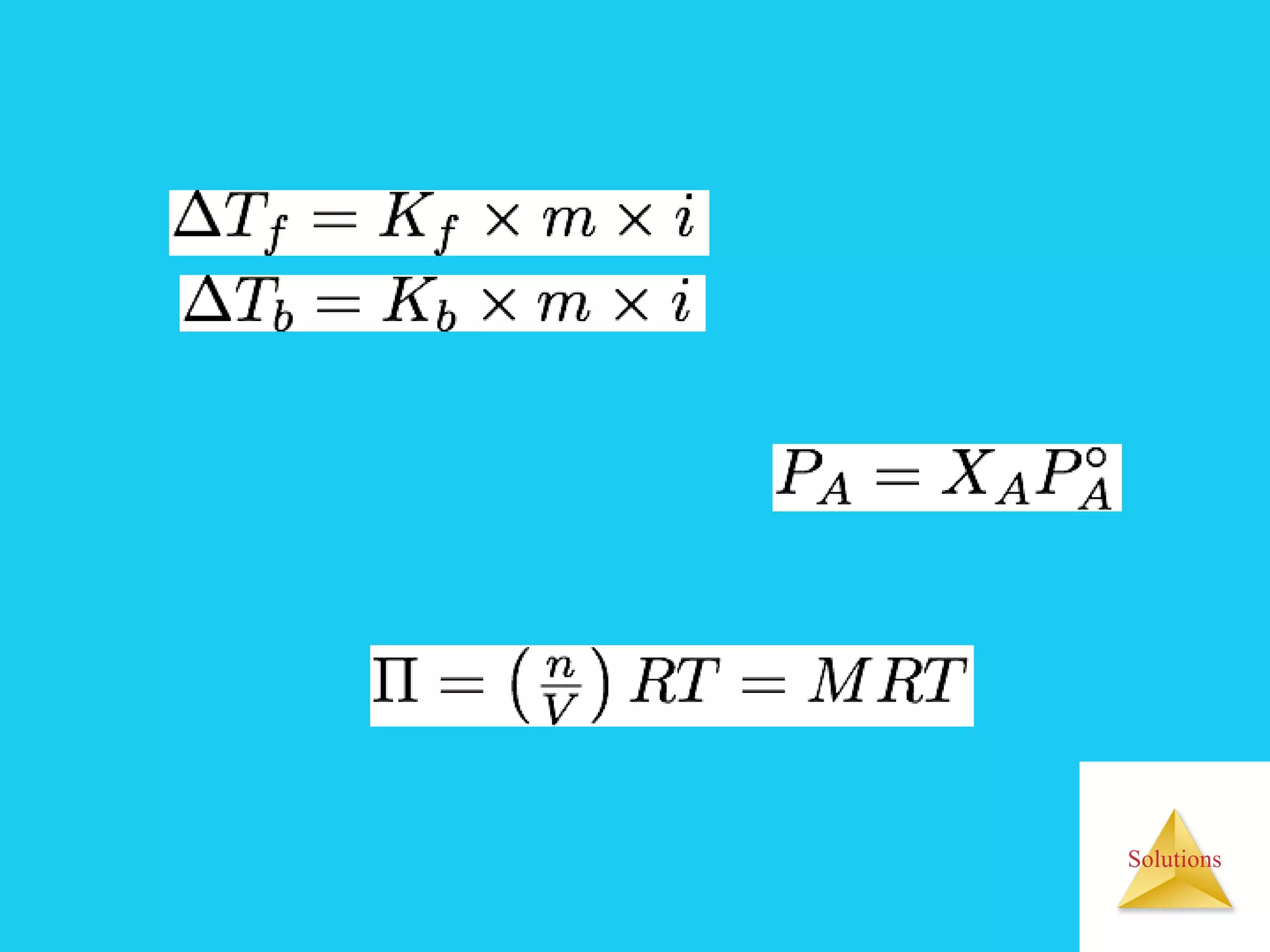This document discusses various topics related to solutions, including:
- How solutions form through interactions between solvent and solute particles
- The enthalpy changes that occur during the dissolution process and how entropy also plays a role
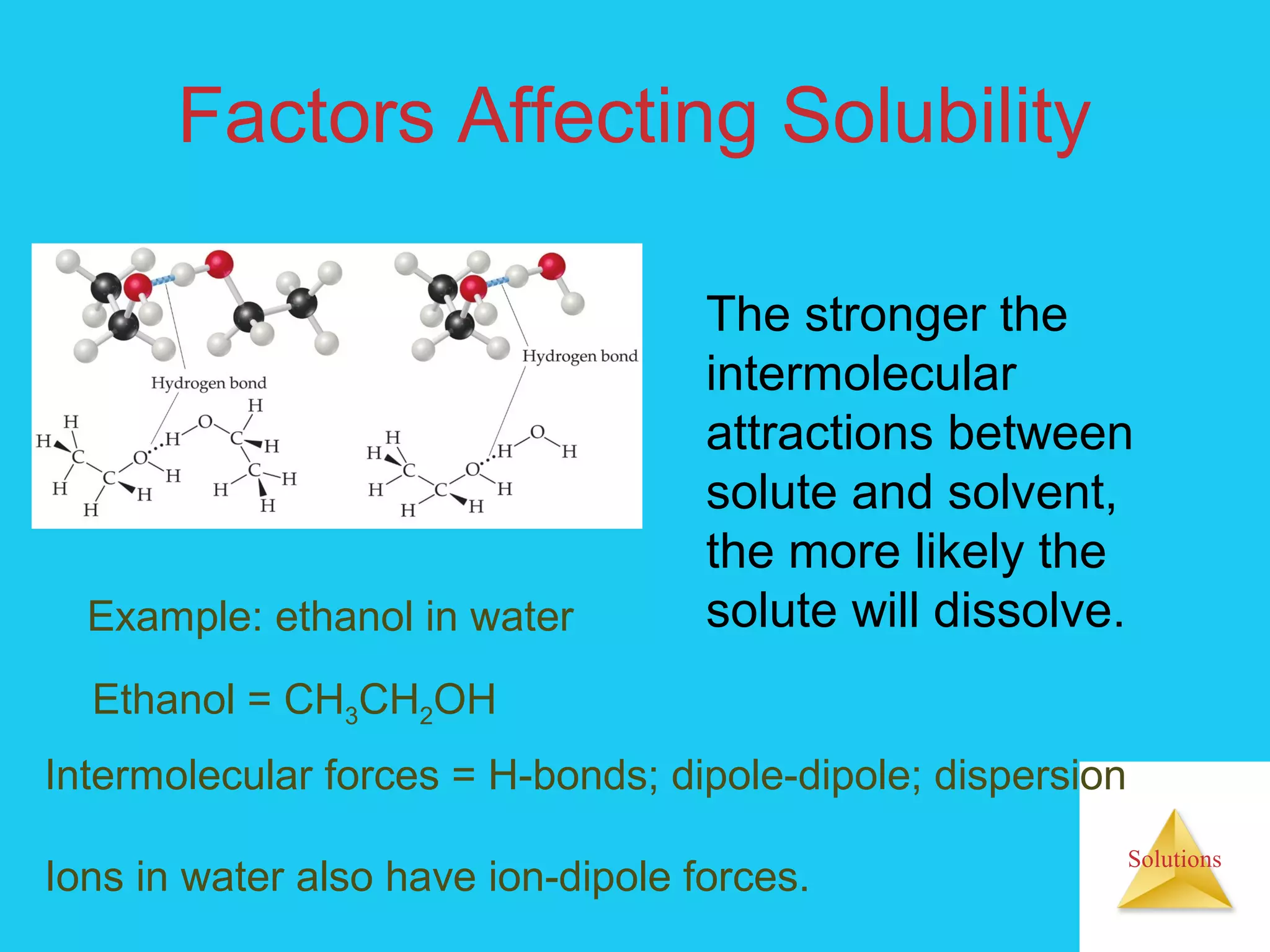
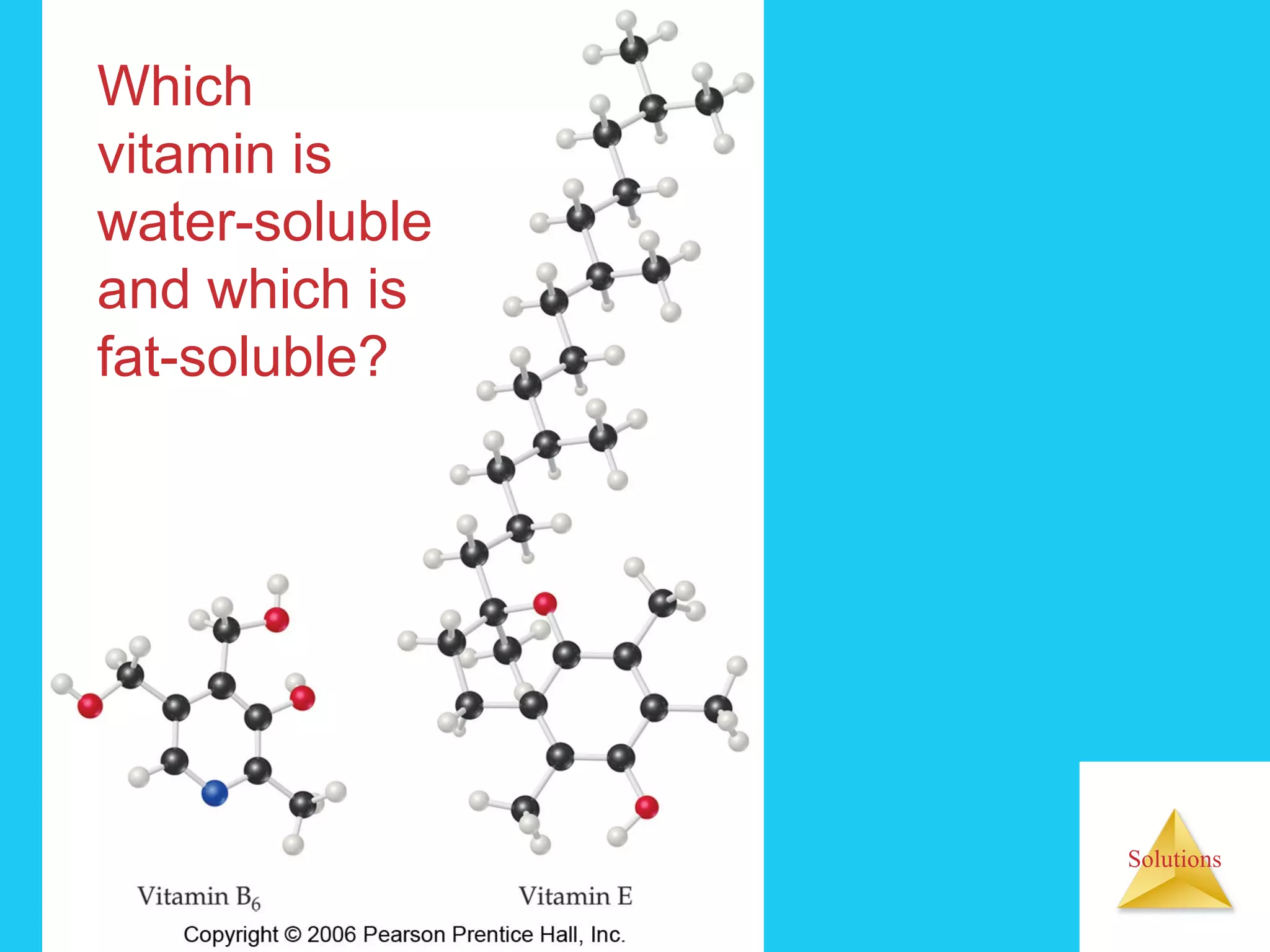
- Factors that affect solubility, such as intermolecular forces
- Different ways of expressing concentration in solutions
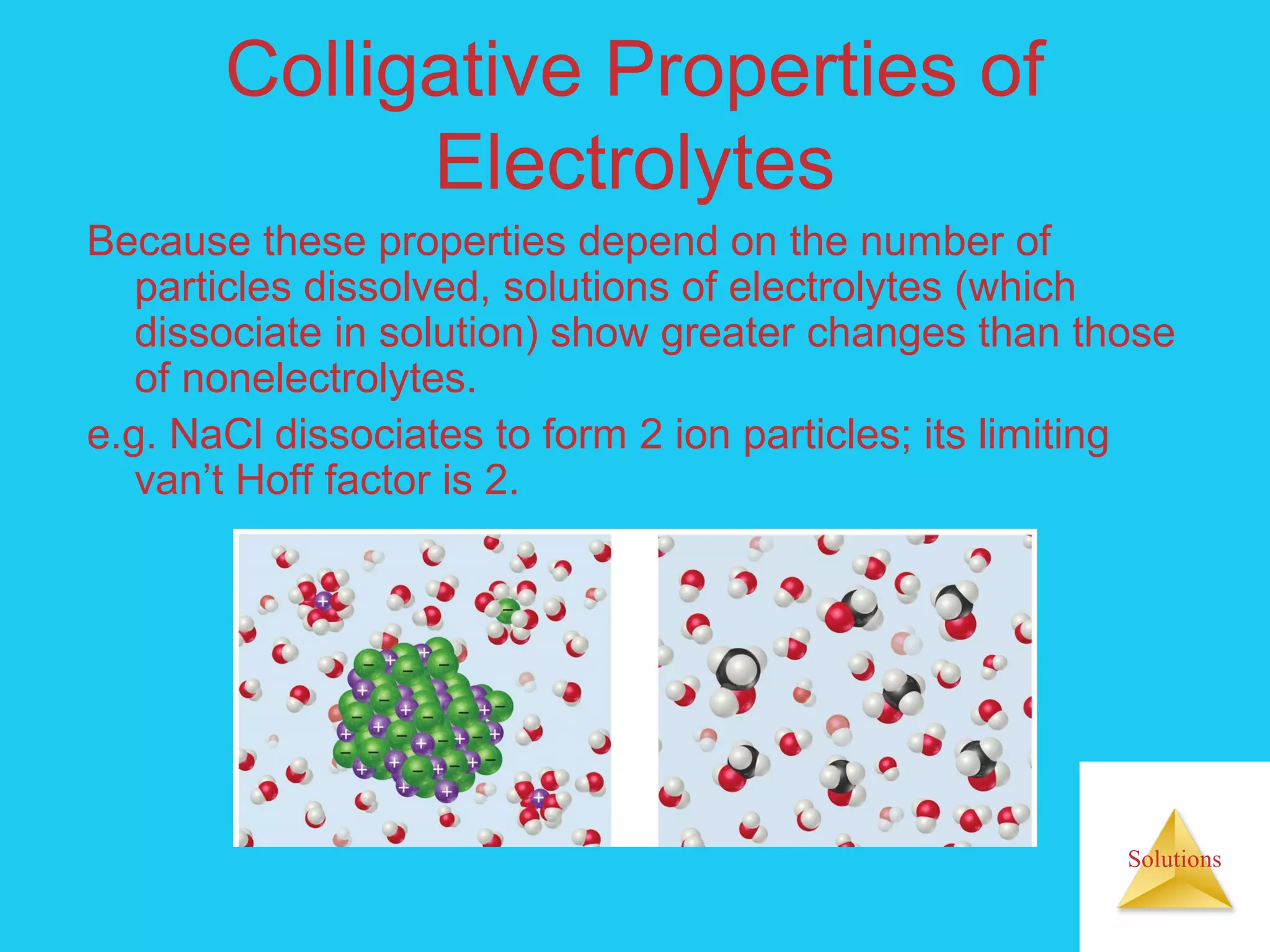
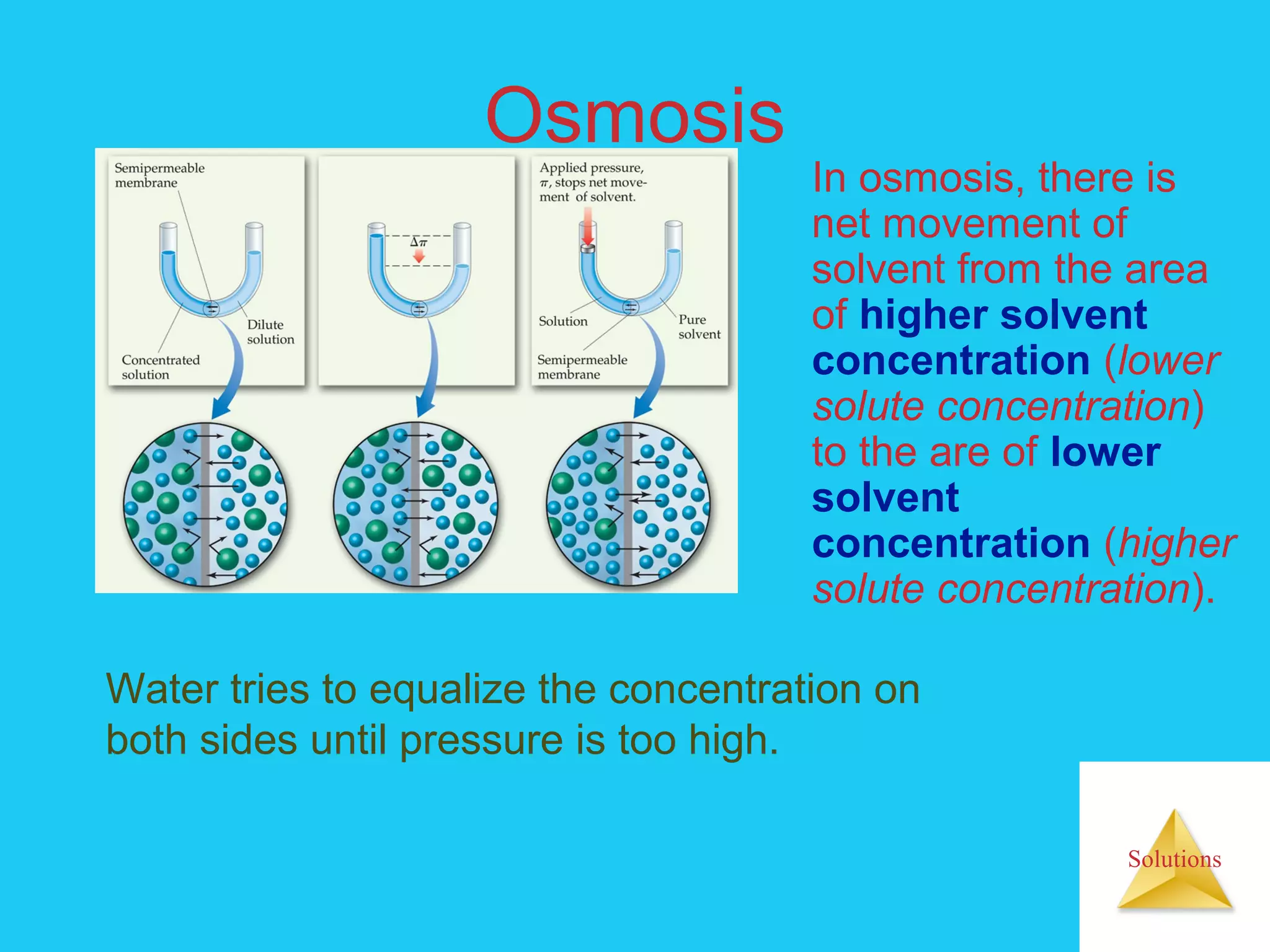
- Colligative properties like boiling point elevation, freezing point depression, and osmotic pressure
- The process of osmosis and how it relates to cell transport