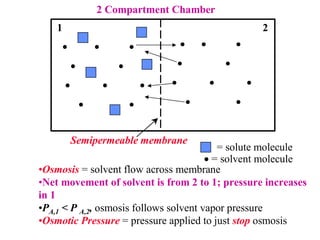
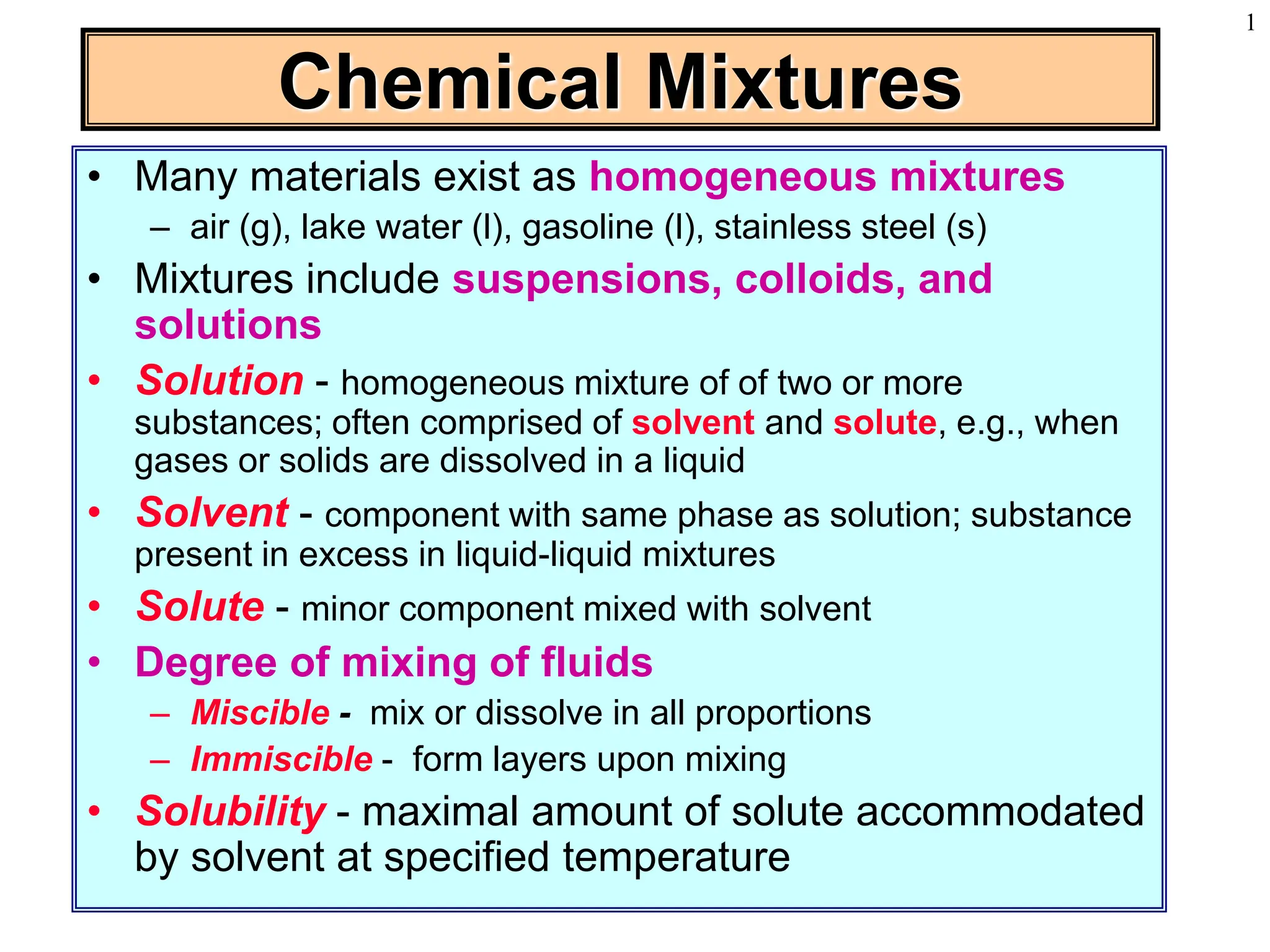
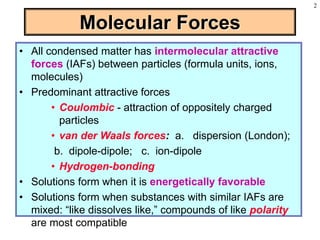
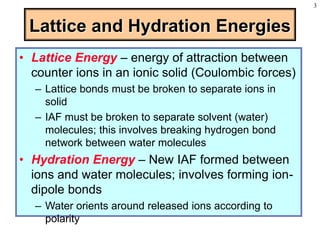
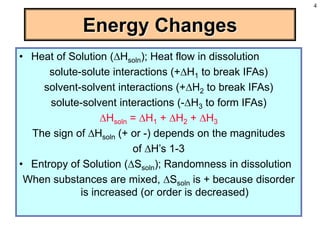
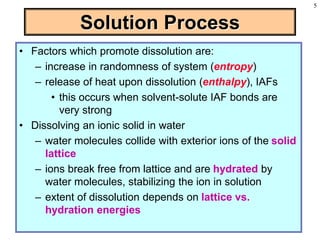
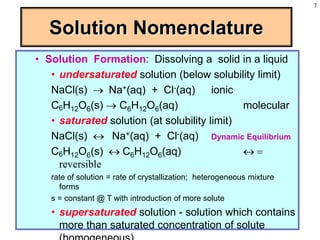
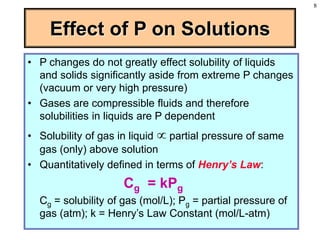
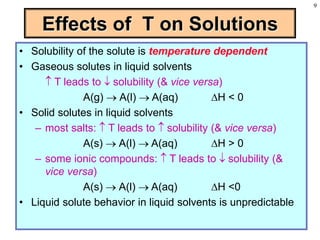
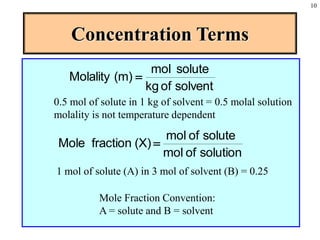
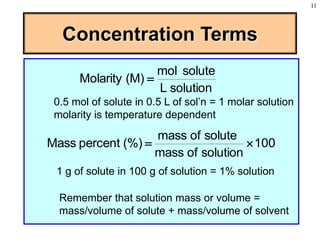
This document discusses chemical mixtures and solutions. It begins by defining key terms like solvent, solute, and miscibility. Intermolecular forces like Coulombic forces, van der Waals forces, and hydrogen bonding determine whether substances will mix. The entropy and enthalpy of the solution process depend on the intermolecular forces between solute-solute, solvent-solvent, and solute-solvent particles. Temperature and pressure can impact gas solubility based on Henry's Law. Concentrated solutions are described using terms like molarity, molality, mole fraction and mass percent. Colligative properties like vapor pressure, freezing point, and boiling point depend only on the number of solute particles regardless of type


![14
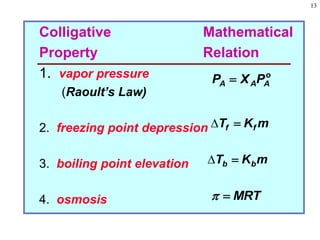
Vapor Pressure, Pv, and Vapor
Pressure Lowering, Pv
• Vapor pressure: partial pressure of vapor above
liquid
• Solution vapor pressure
– Examples include volatile solvent with non-volatile
solute and volatile solute and volatile solvent
– In example of non-volatile solute, vapor pressure
of solution proportional to concentration of solvent
Pv,A [solvent A] = XAPo
A
As solvent concentration decreases, Pv decreases
– In example of volatile solute (B) and solvent (A)
PT = PA + PB = XAPo
A + XBPo
B](https://image.slidesharecdn.com/1solutionchemistry-231025234335-5898c20d/85/1_Solution-Chemistry-ppt-14-320.jpg)