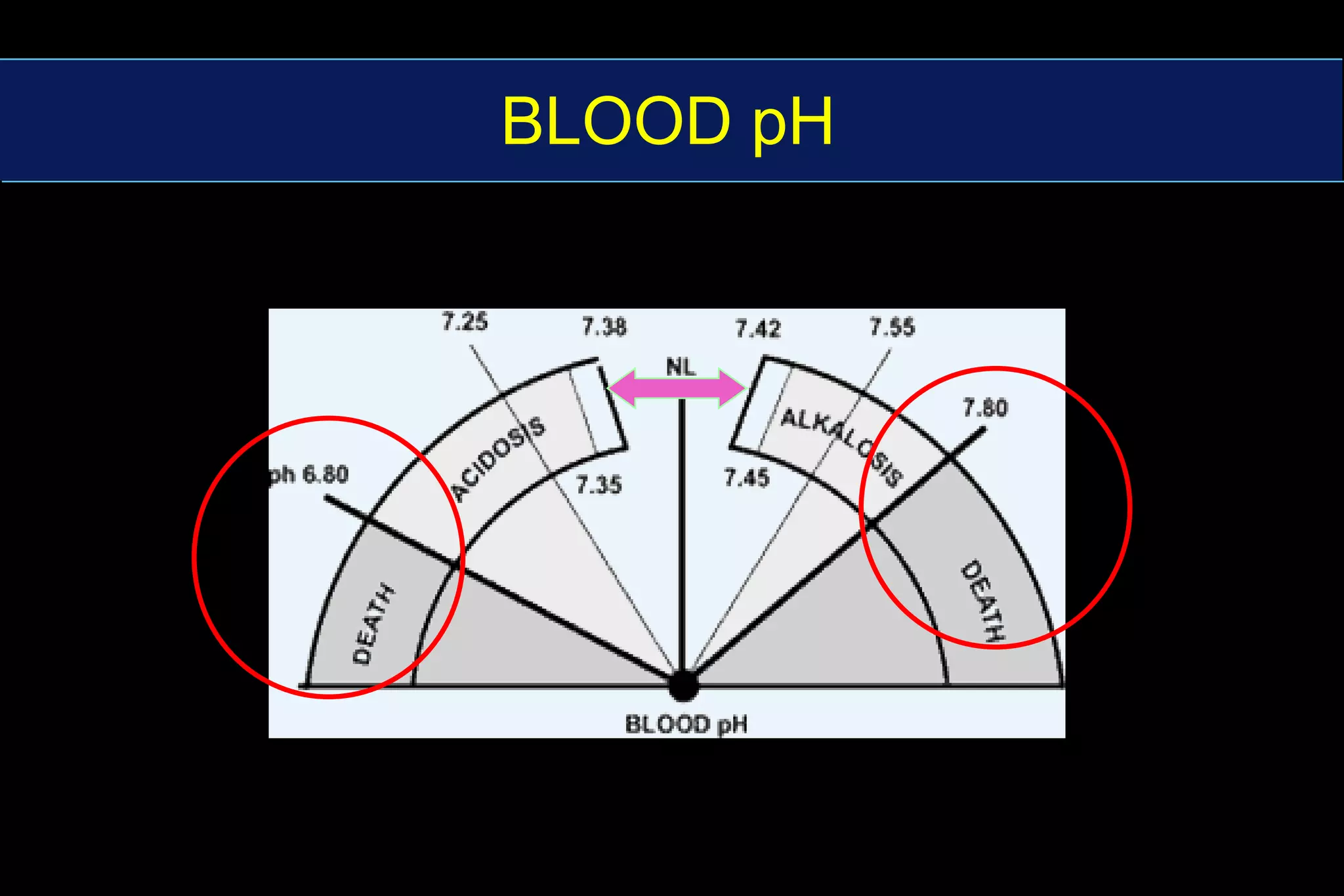
This document summarizes blood gas analysis and acid-base balance. It describes how pH is maintained between 7.36-7.44 through bicarbonate and phosphate buffer systems. Respiratory and metabolic acidosis and alkalosis are explained in relation to changes in CO2 and bicarbonate levels. Key factors in analyzing acid-base disturbances including anion gap, predicted respiratory pH, and metabolic components are outlined. Different types of acid-base disorders and their diagnoses are also summarized.





















![Bicarbonate buffer systems:CO2 + H2O H2CO3 H+ + HCO3-pK = 6.1 [HCO3-] = 24 mmol/L](https://image.slidesharecdn.com/blood-gas-analysis4231/75/Blood-Gas-Analysis-23-2048.jpg)







![Renal buffering mechanisms Renal - kidney excretes H+ and replenishes [HCO3-] .But, this is a slow process taking hours to days.](https://image.slidesharecdn.com/blood-gas-analysis4231/75/Blood-Gas-Analysis-31-2048.jpg)





![RESPIRATORY ACIDOSISH2O + CO2 H2CO3 H+ + HCO3- Cause - hypoventilationRetention of CO2 Drives equation rightwardIncreases both [H+] and [HCO3-]](https://image.slidesharecdn.com/blood-gas-analysis4231/75/Blood-Gas-Analysis-37-2048.jpg)


![RESPIRATORY ALKALOSISH2O + CO2 H2CO3 H+ + HCO3- 2. Respiratory Alkalosis cause - hyperventilationBlows off CO2 Drives equation leftward decreasing both [H+] and [HCO3-]](https://image.slidesharecdn.com/blood-gas-analysis4231/75/Blood-Gas-Analysis-40-2048.jpg)




























![Anion GapAG = [Na + ] - [Cl ‾ + HCO3‾ ]• AG represents unaccounted for anions (R ‾ )• Normal anion gap = 10](https://image.slidesharecdn.com/blood-gas-analysis4231/75/Blood-Gas-Analysis-69-2048.jpg)














