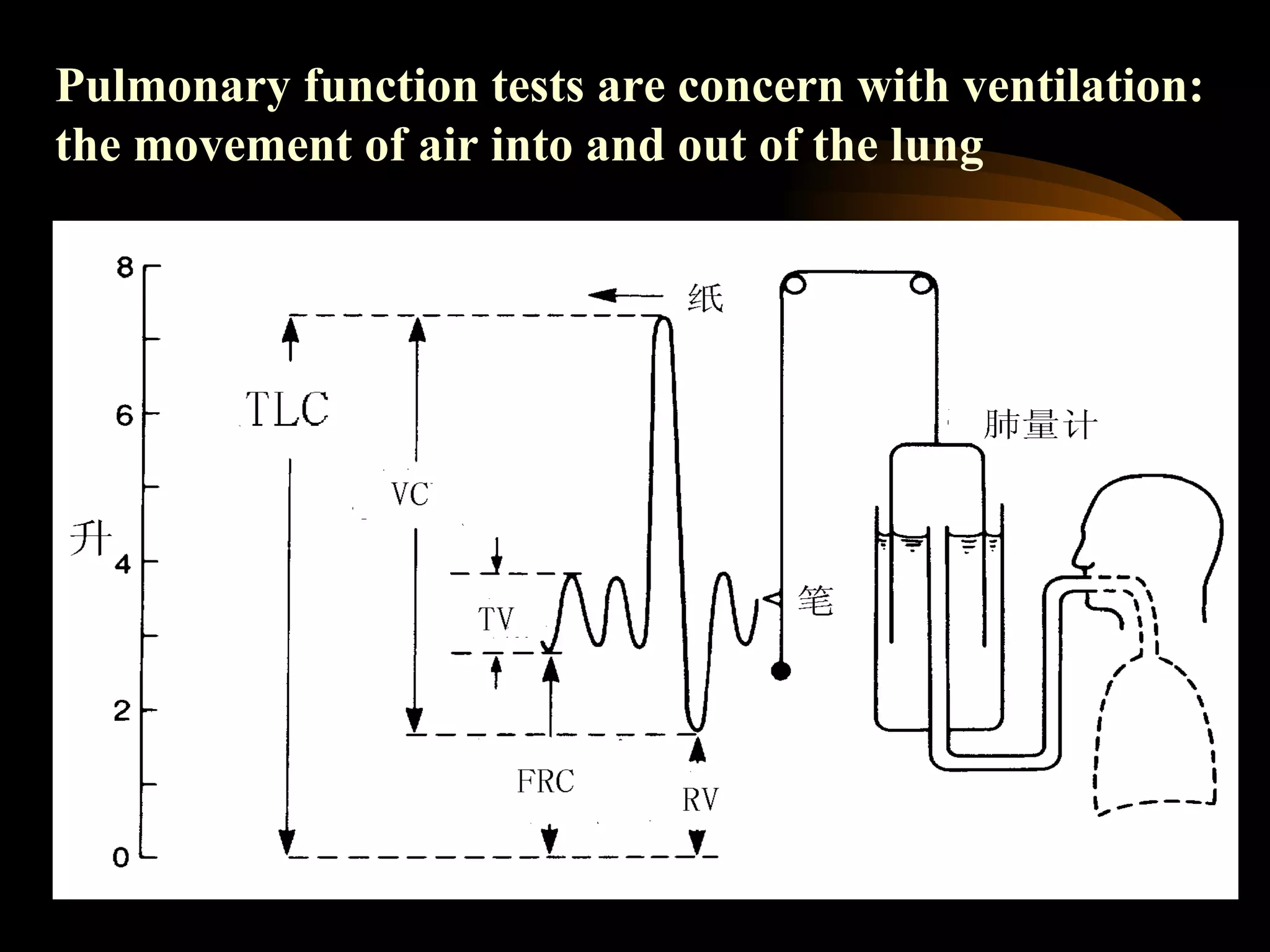
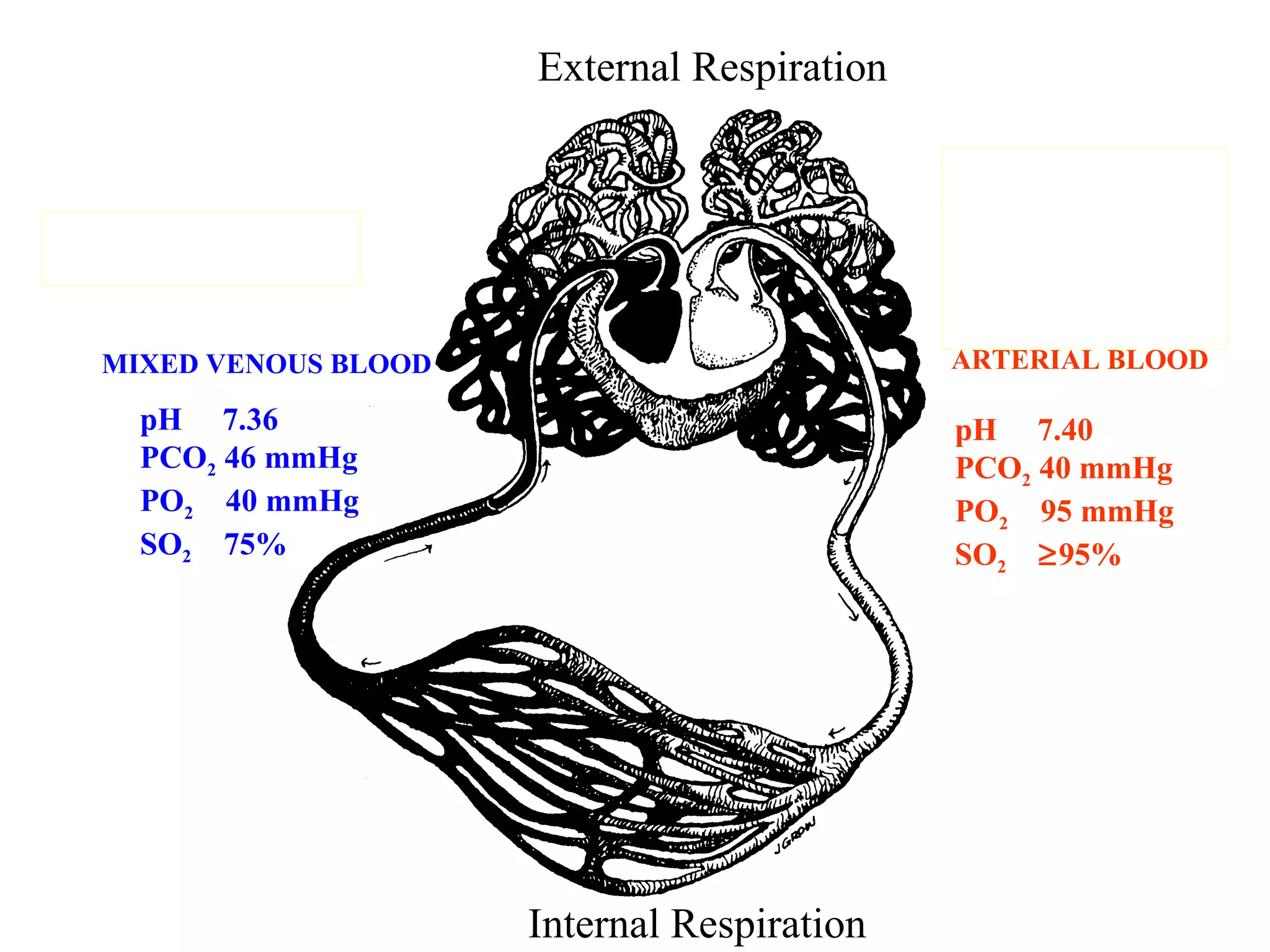
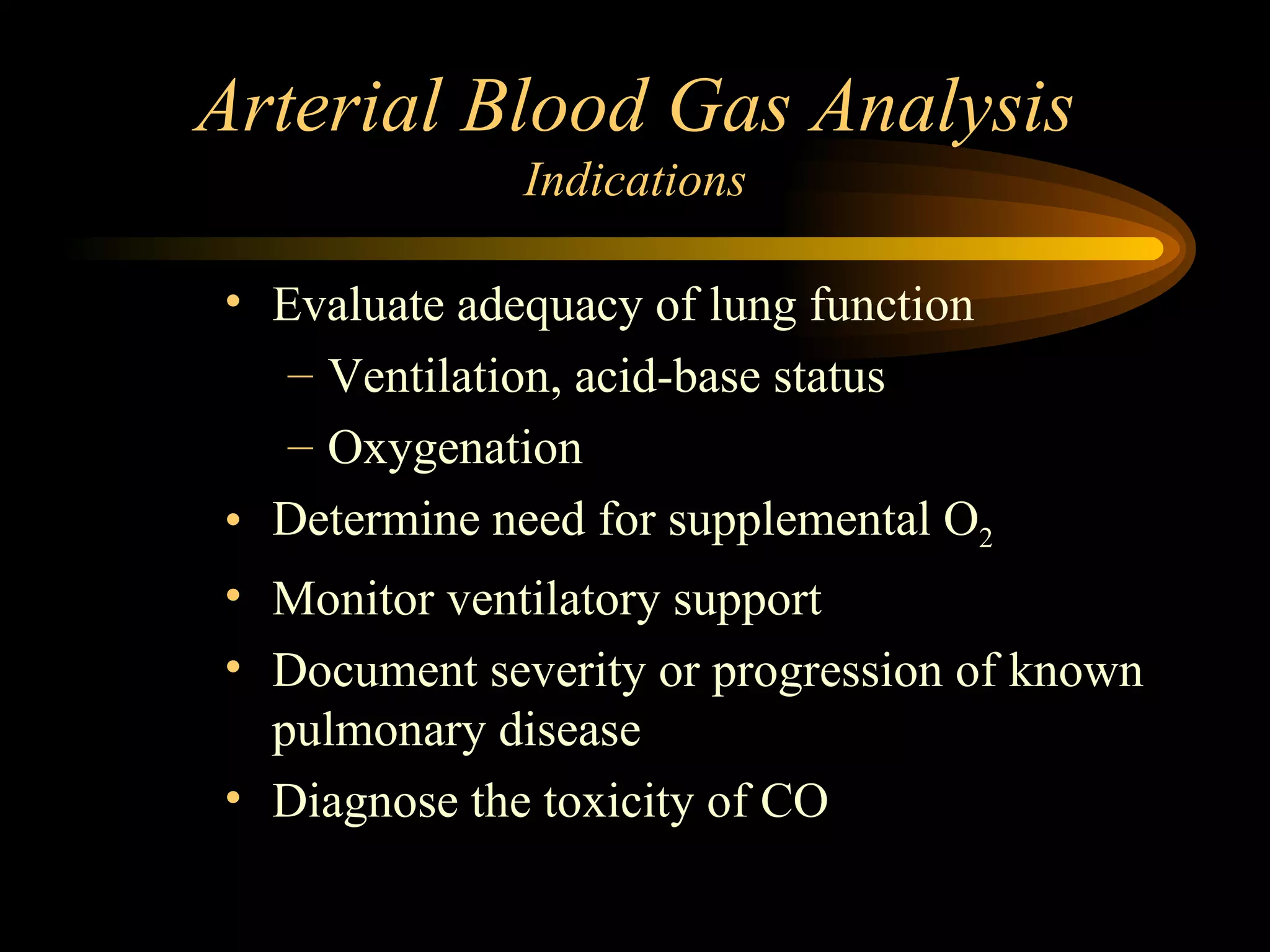
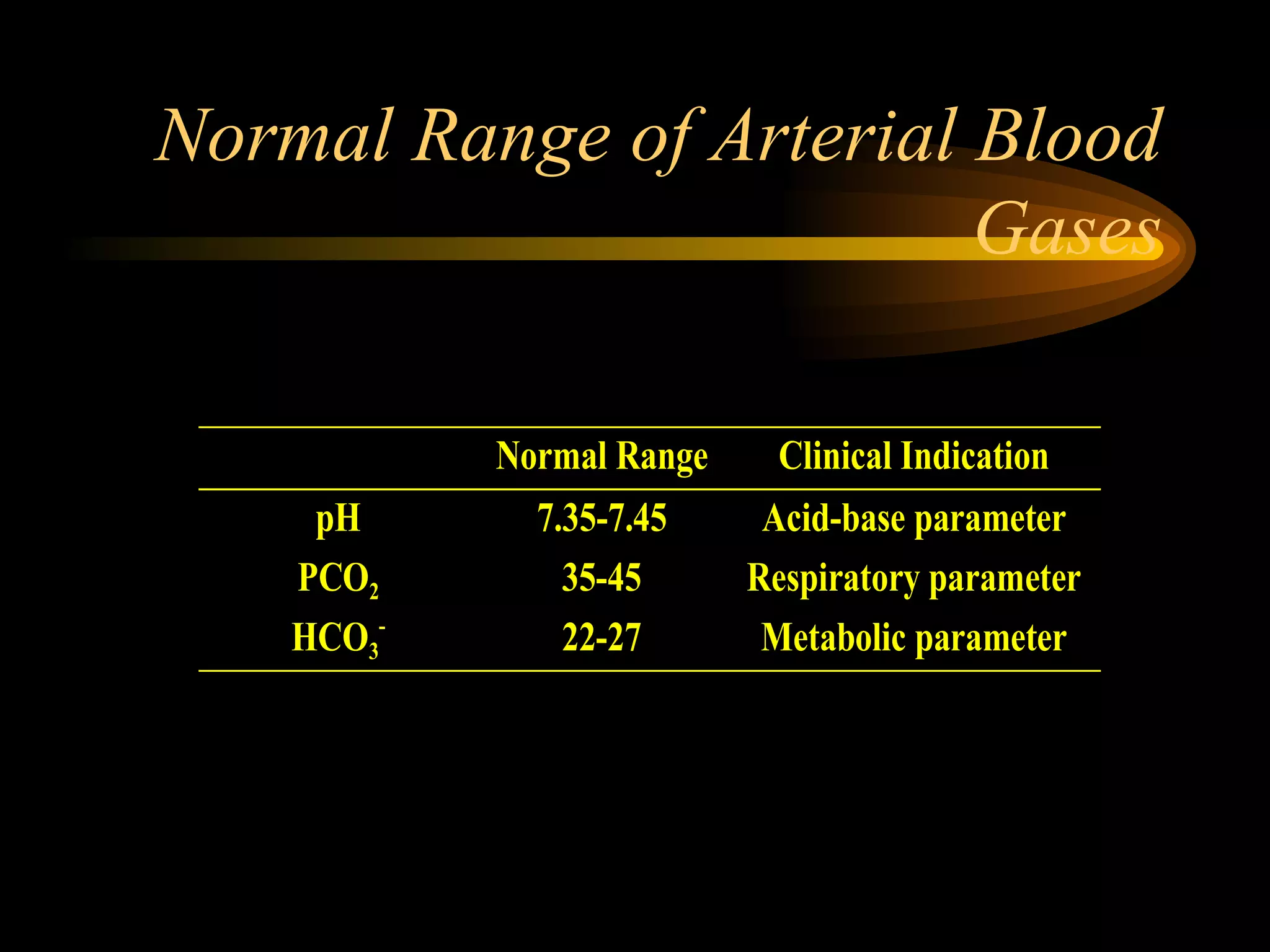
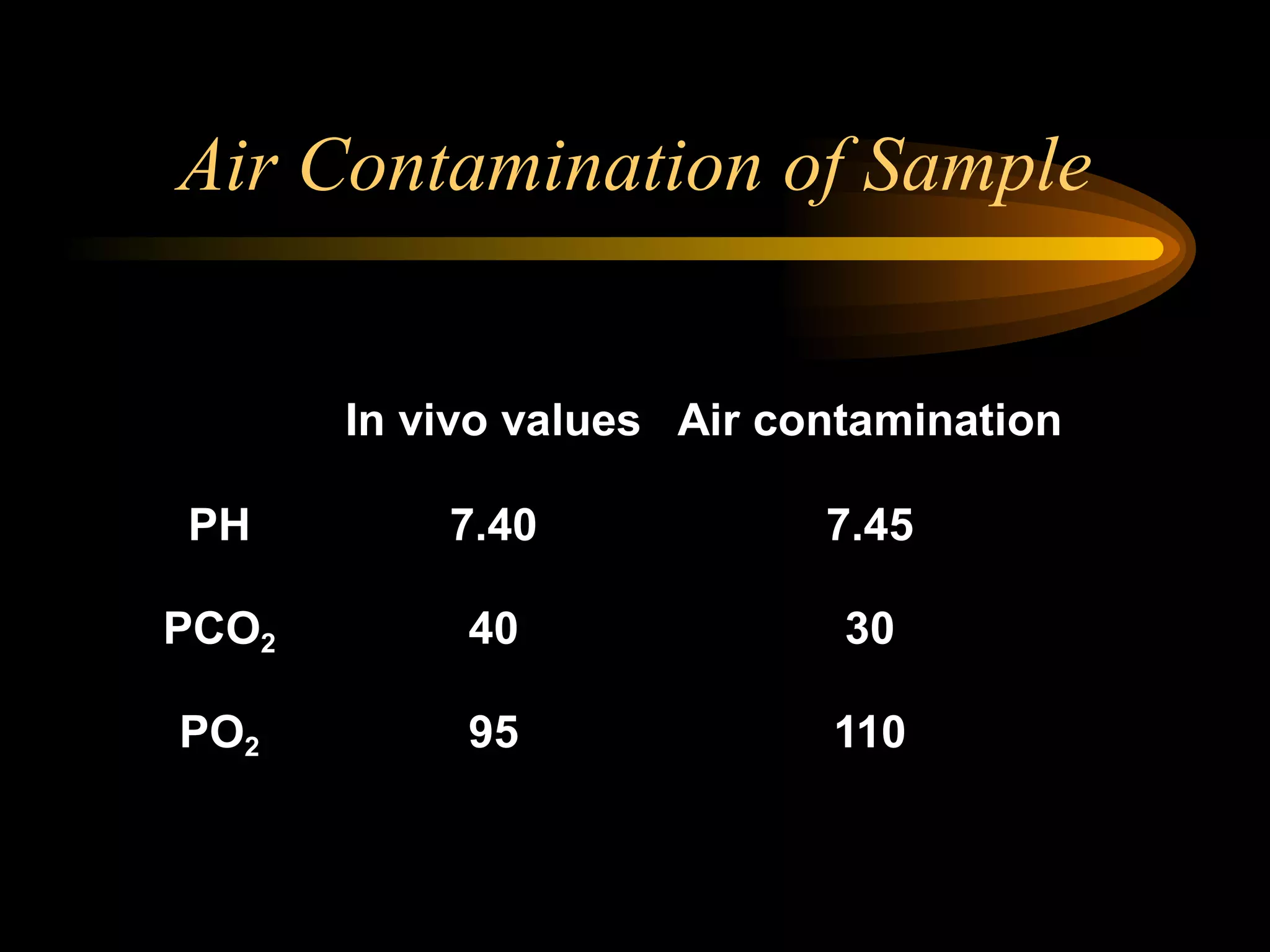
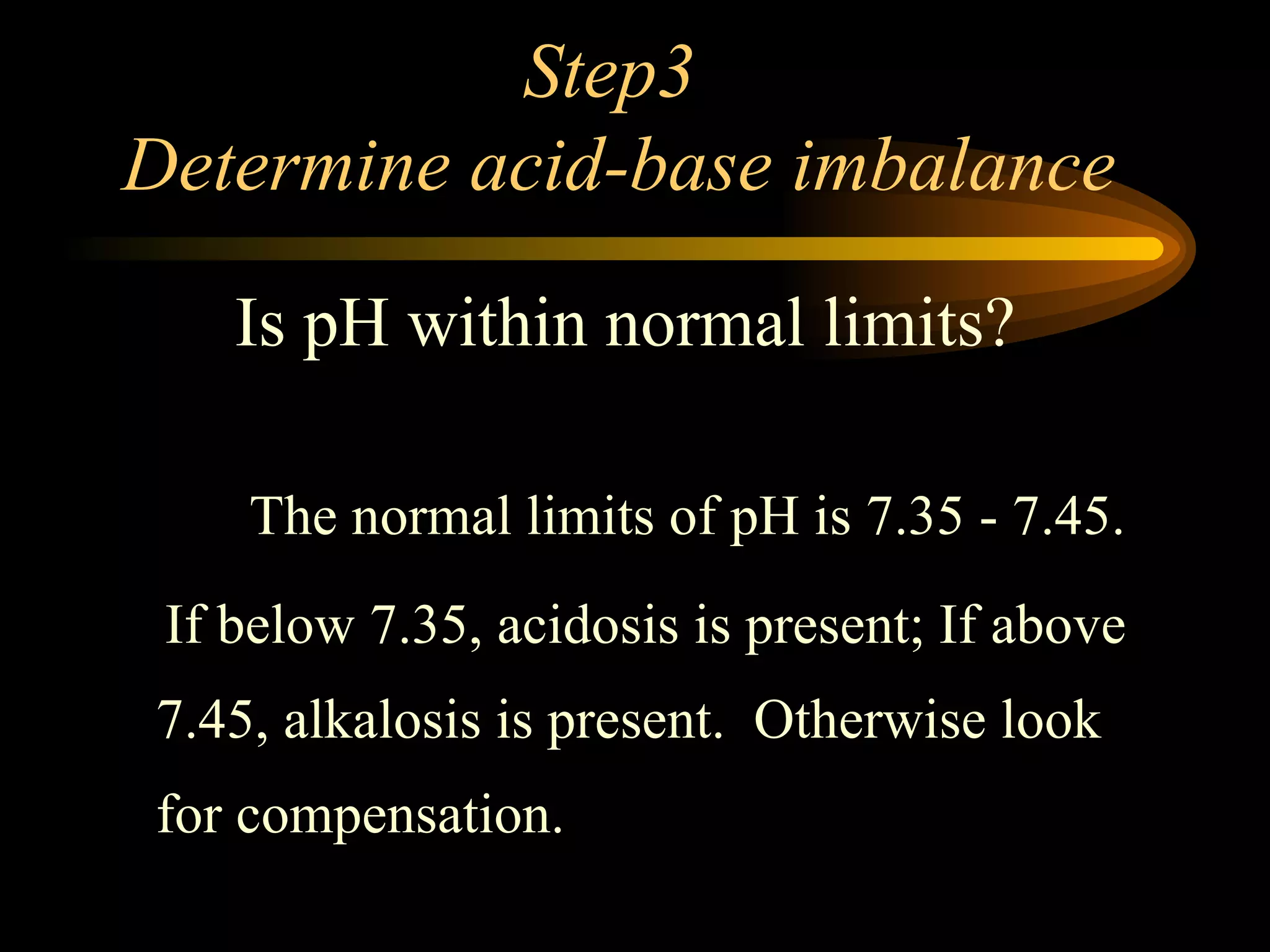
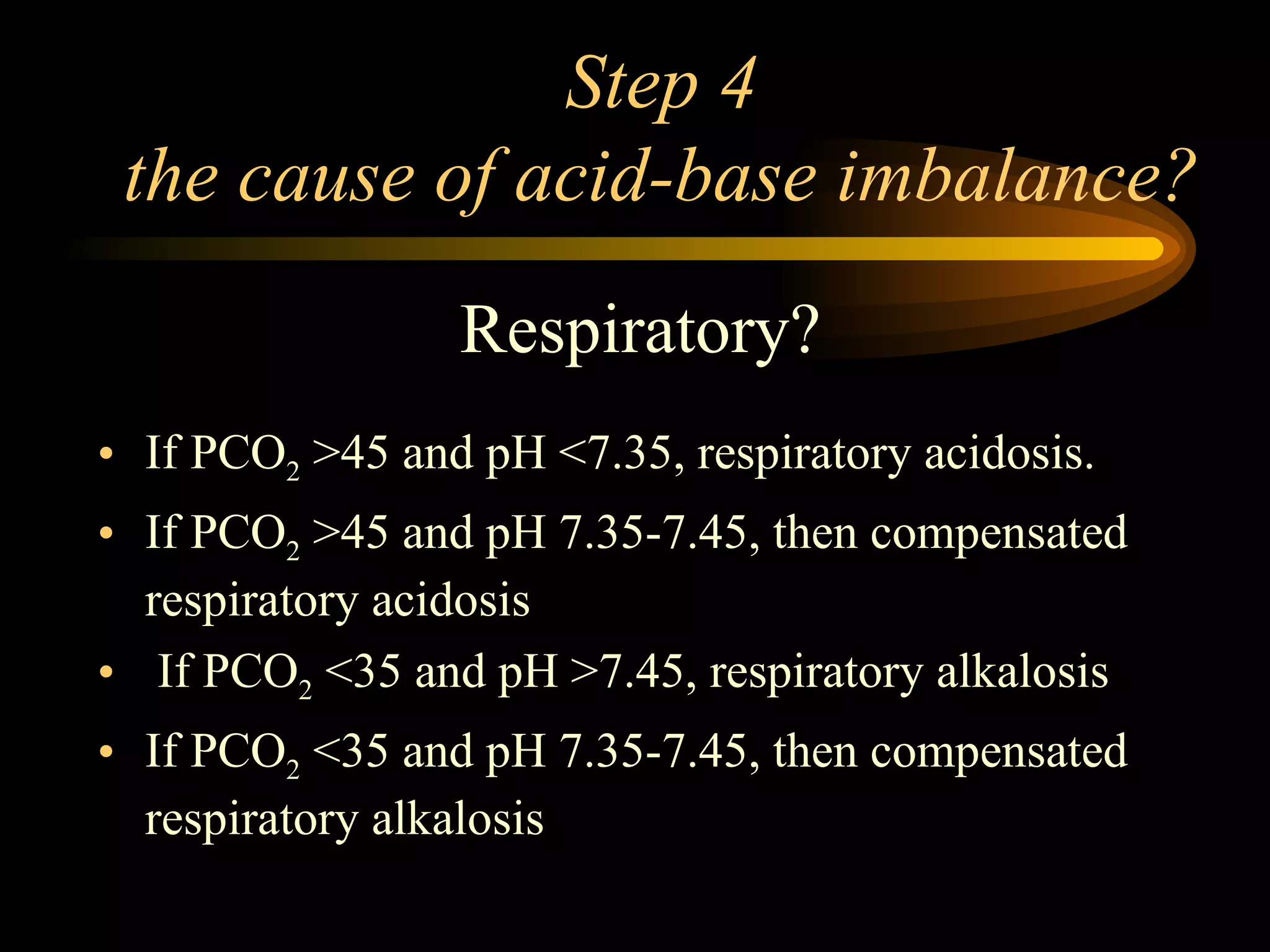
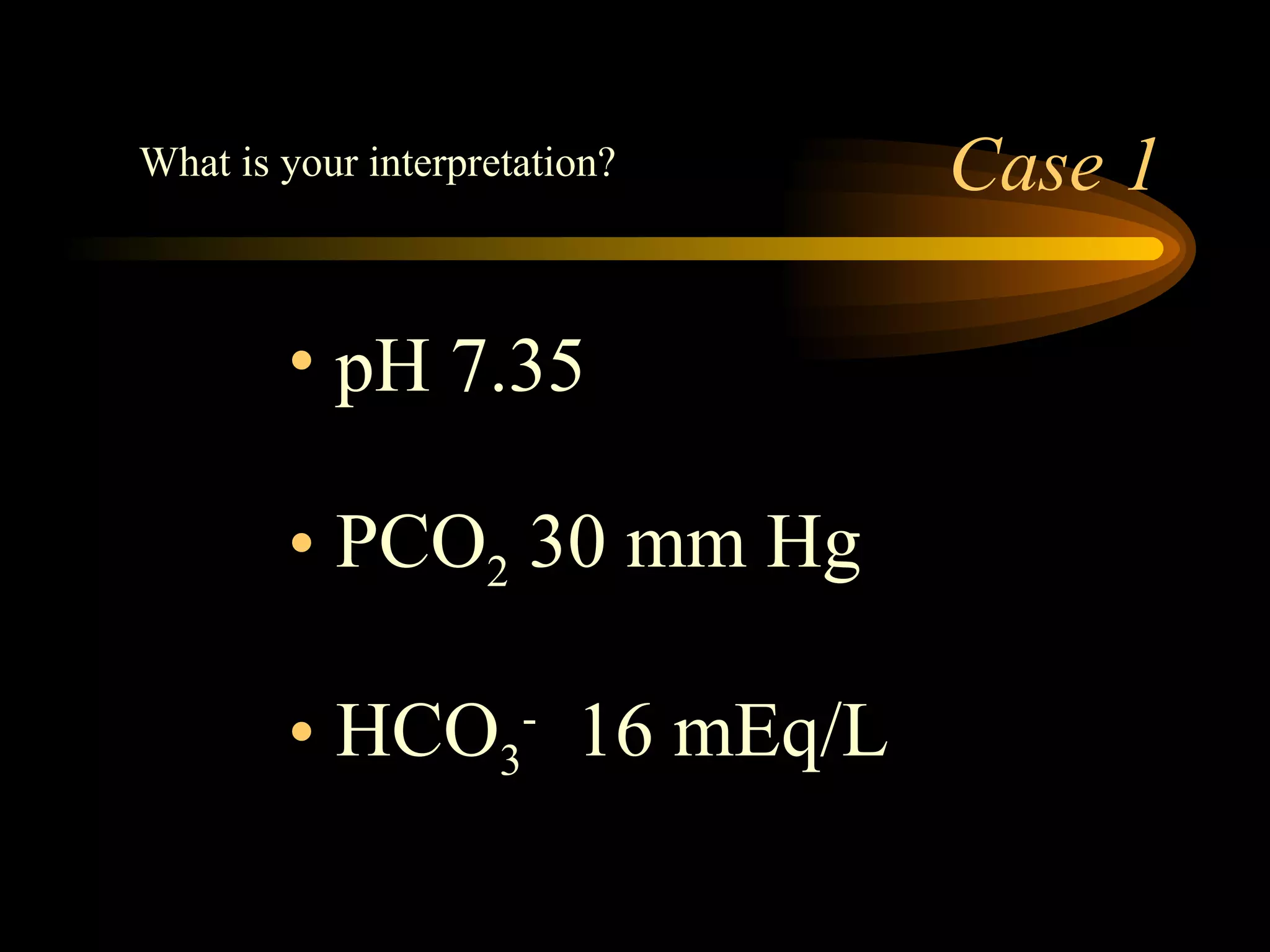
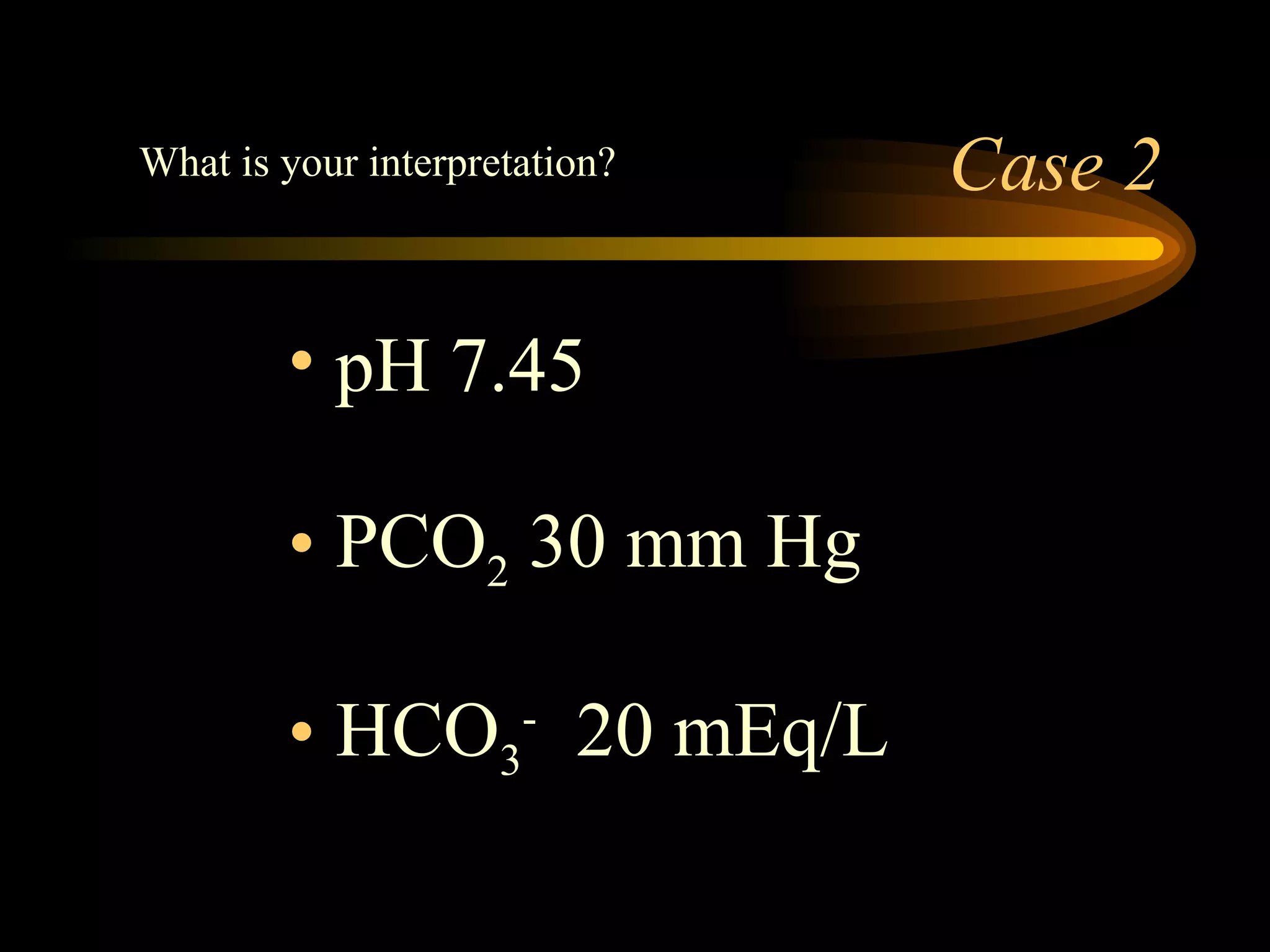
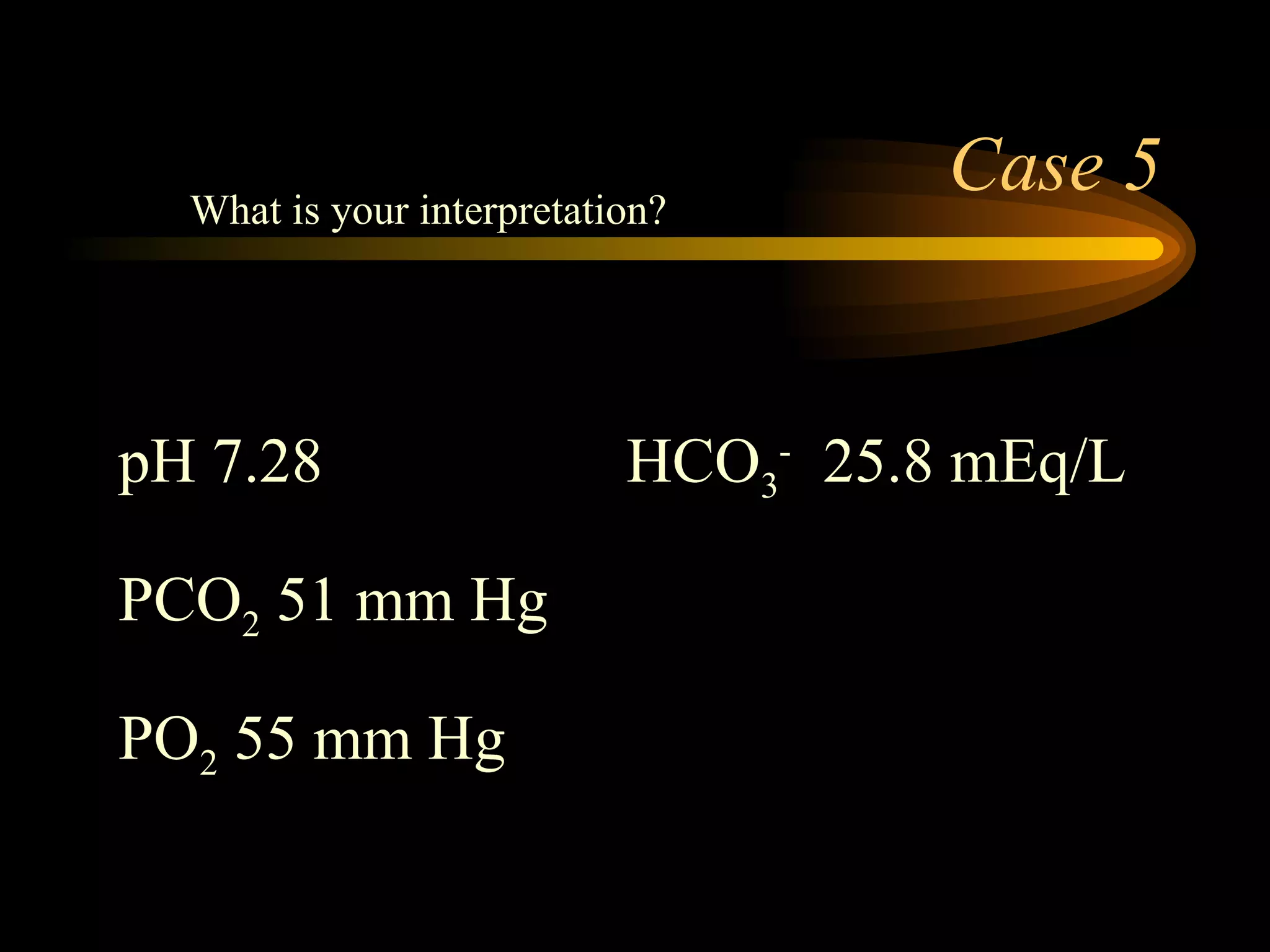
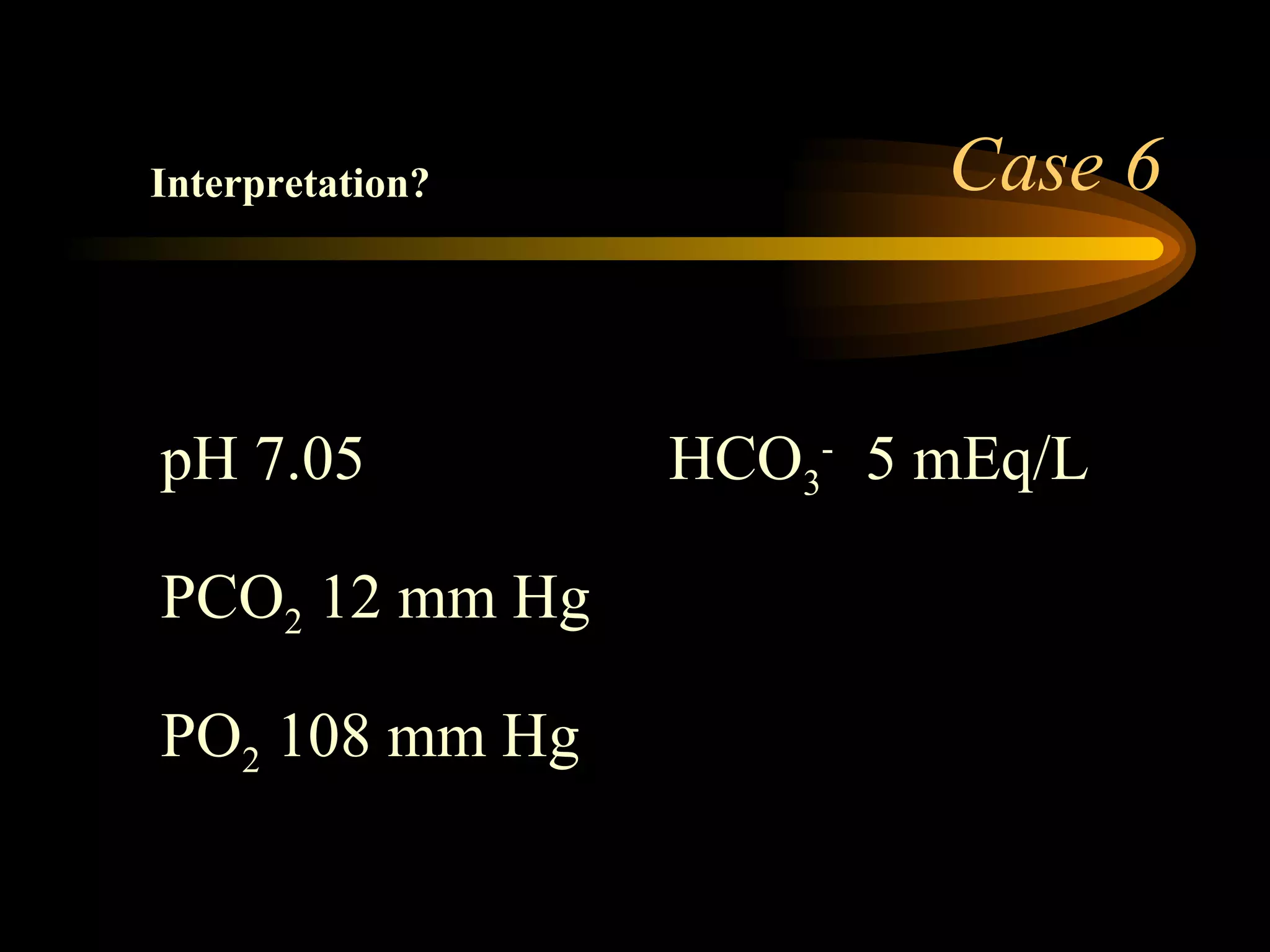
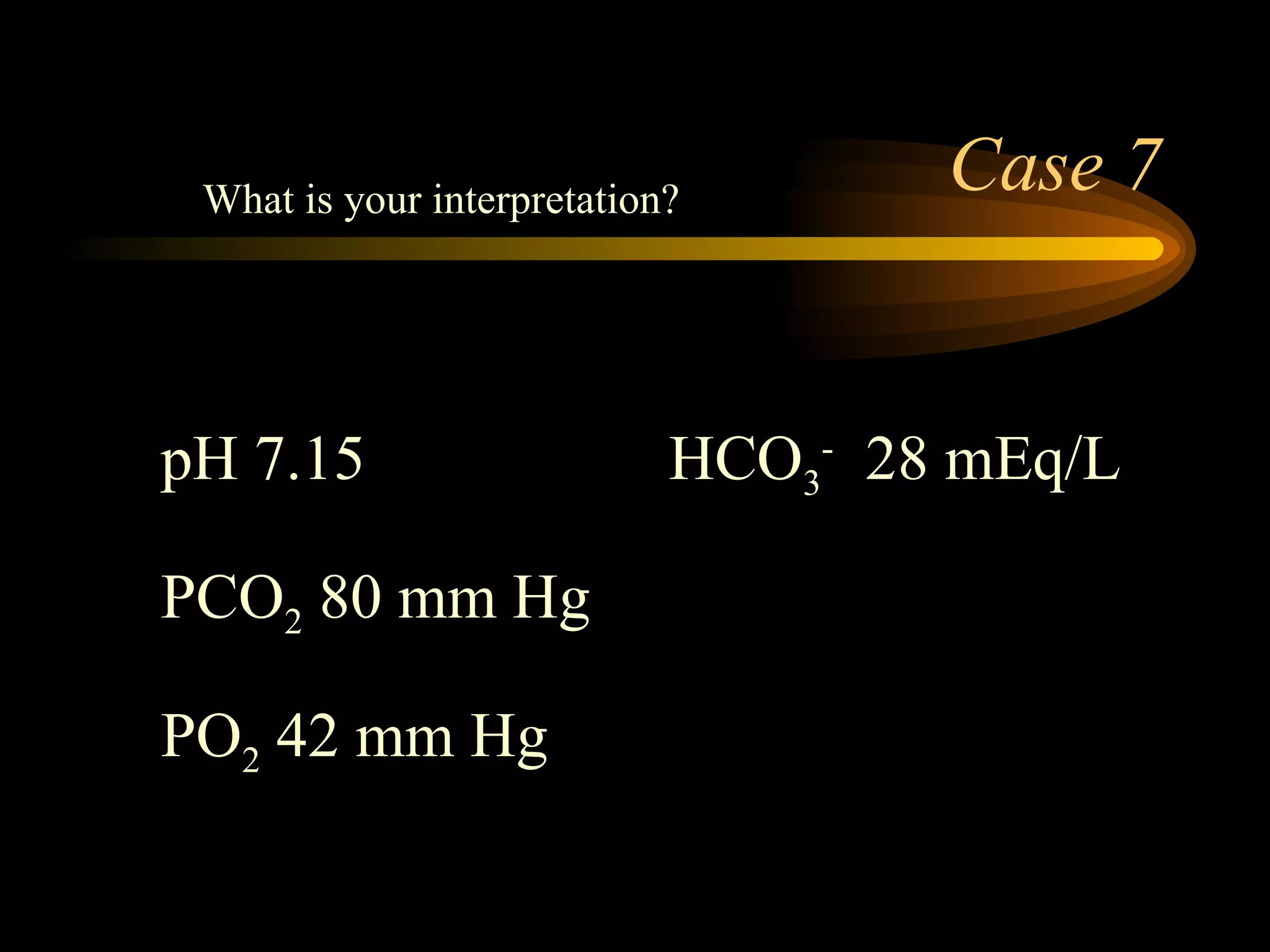
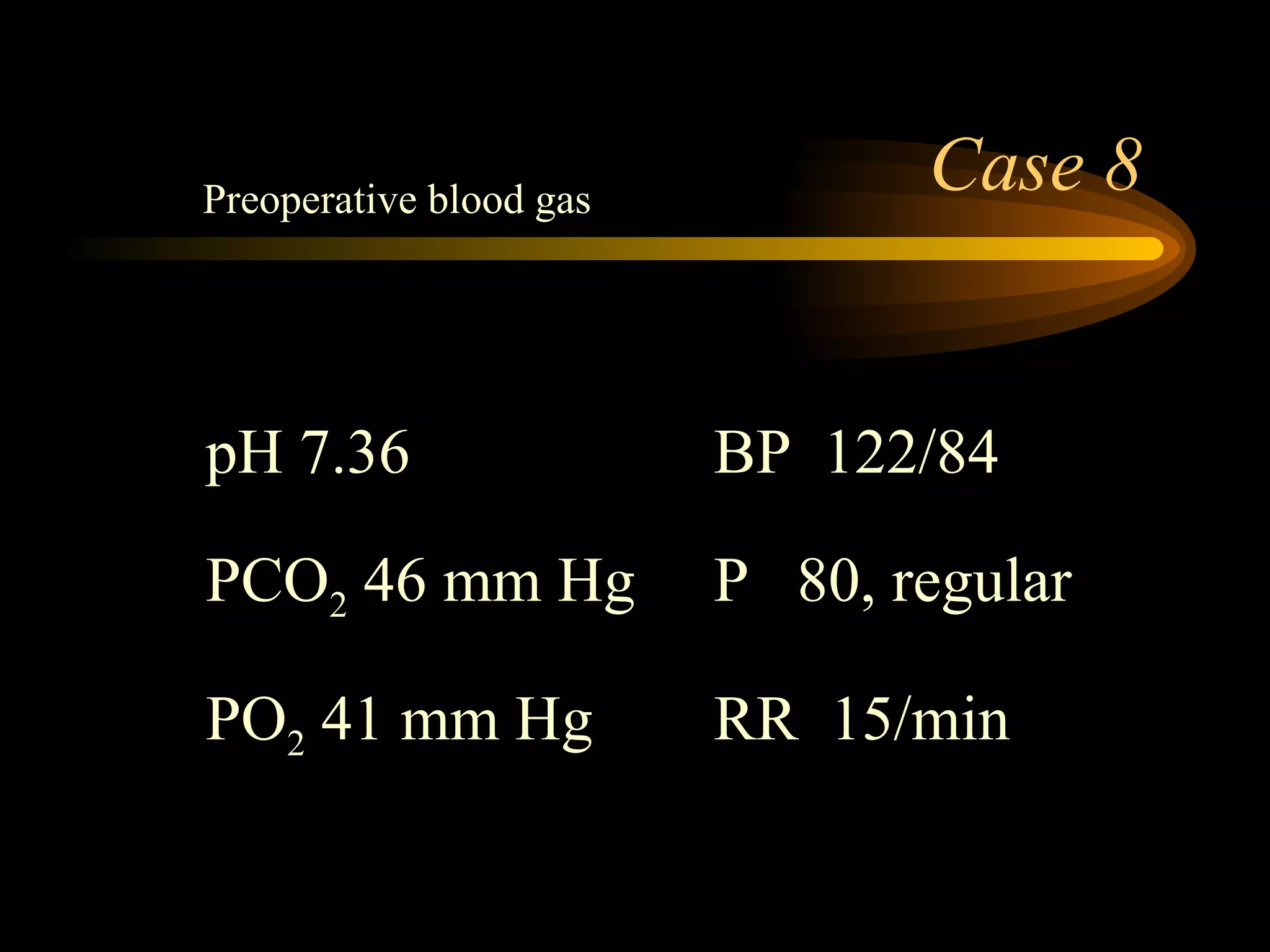
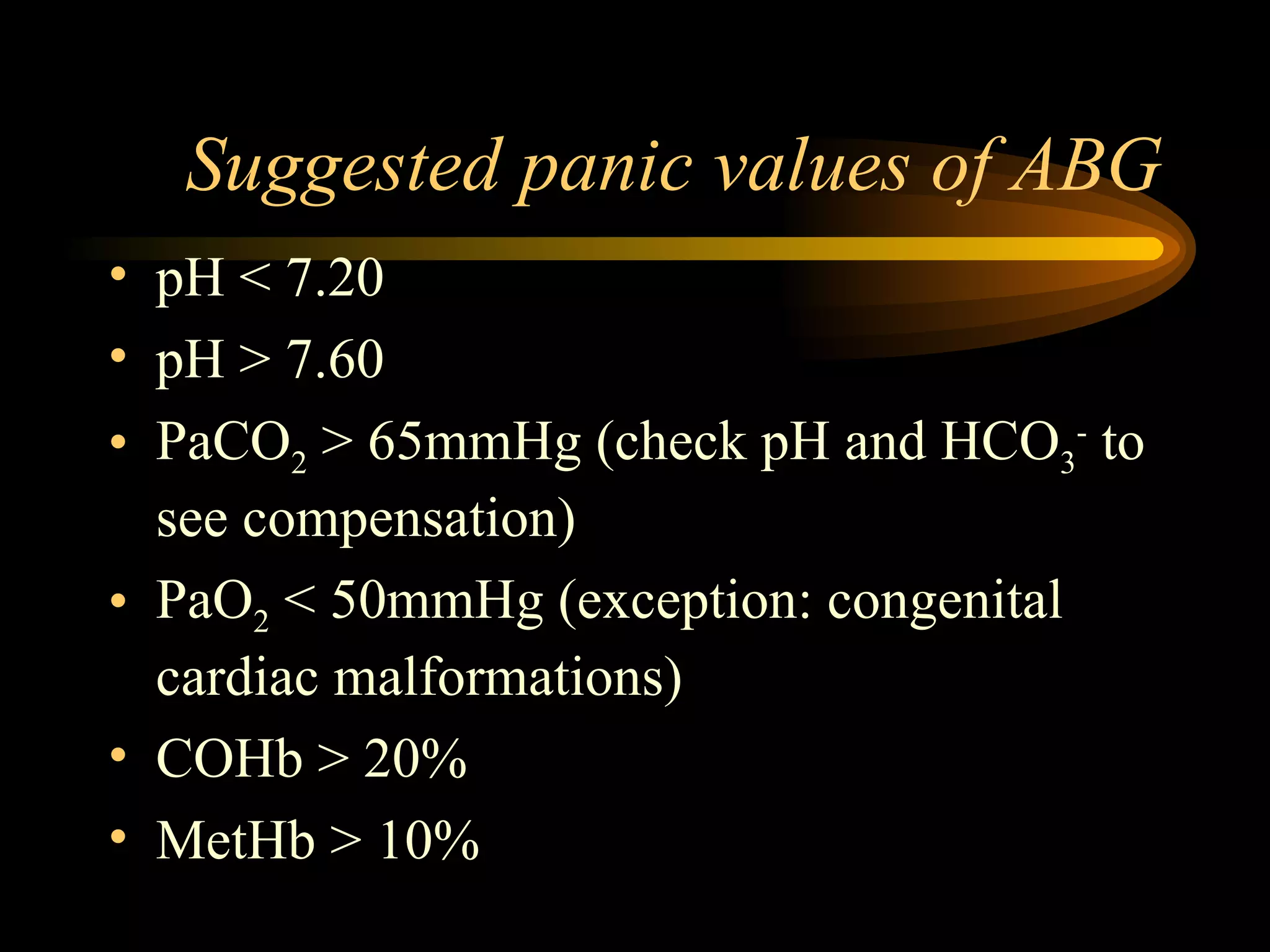
The document discusses arterial blood gas analysis, which provides information on oxygenation, ventilation, and acid-base balance. It outlines the key parameters measured in an ABG test and strategies for interpreting the results, including evaluating for respiratory or metabolic causes of acid-base imbalances and hypoxemia. Several case studies are presented to demonstrate interpreting ABG values in the context of patients' clinical presentations.