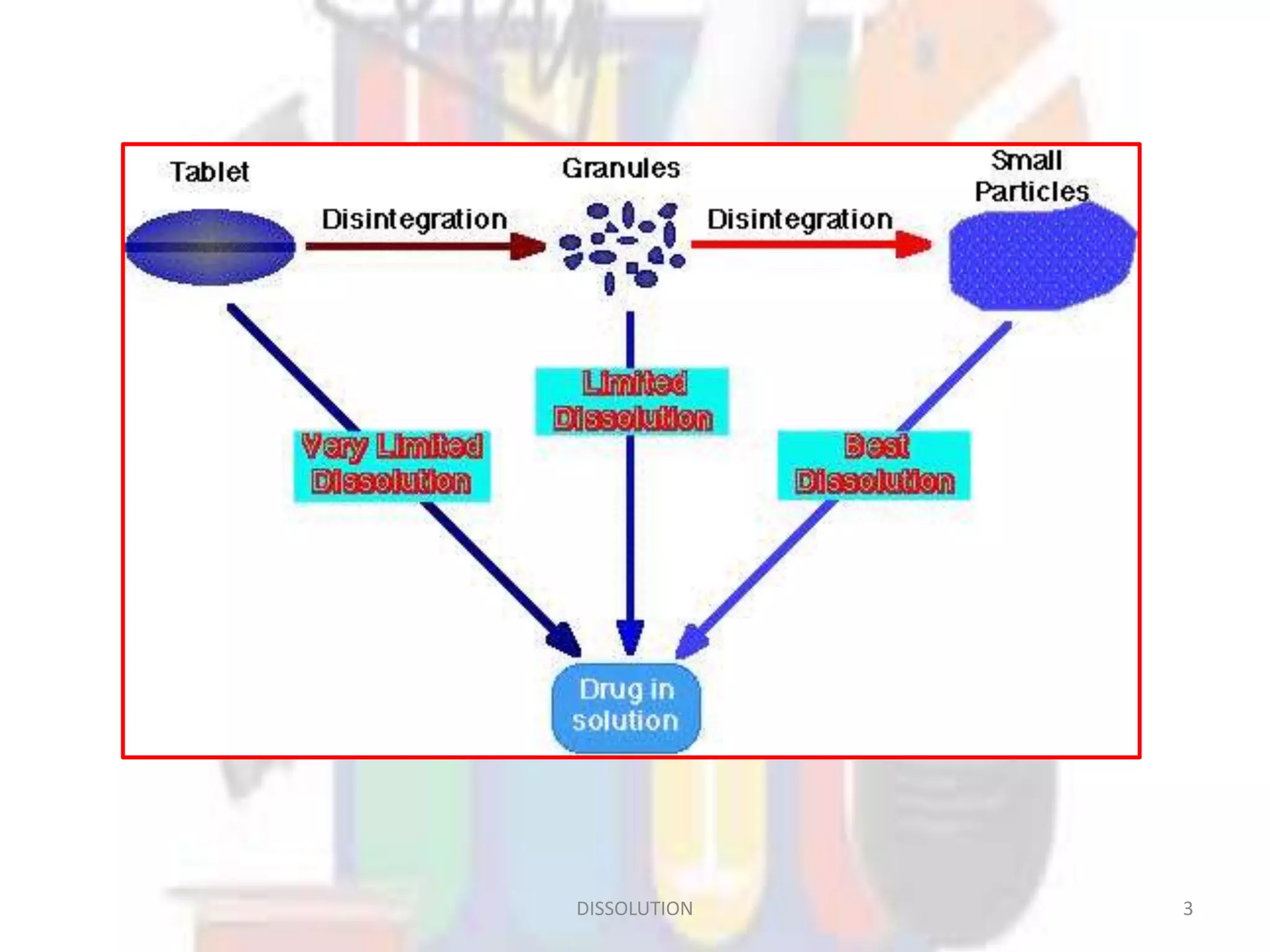
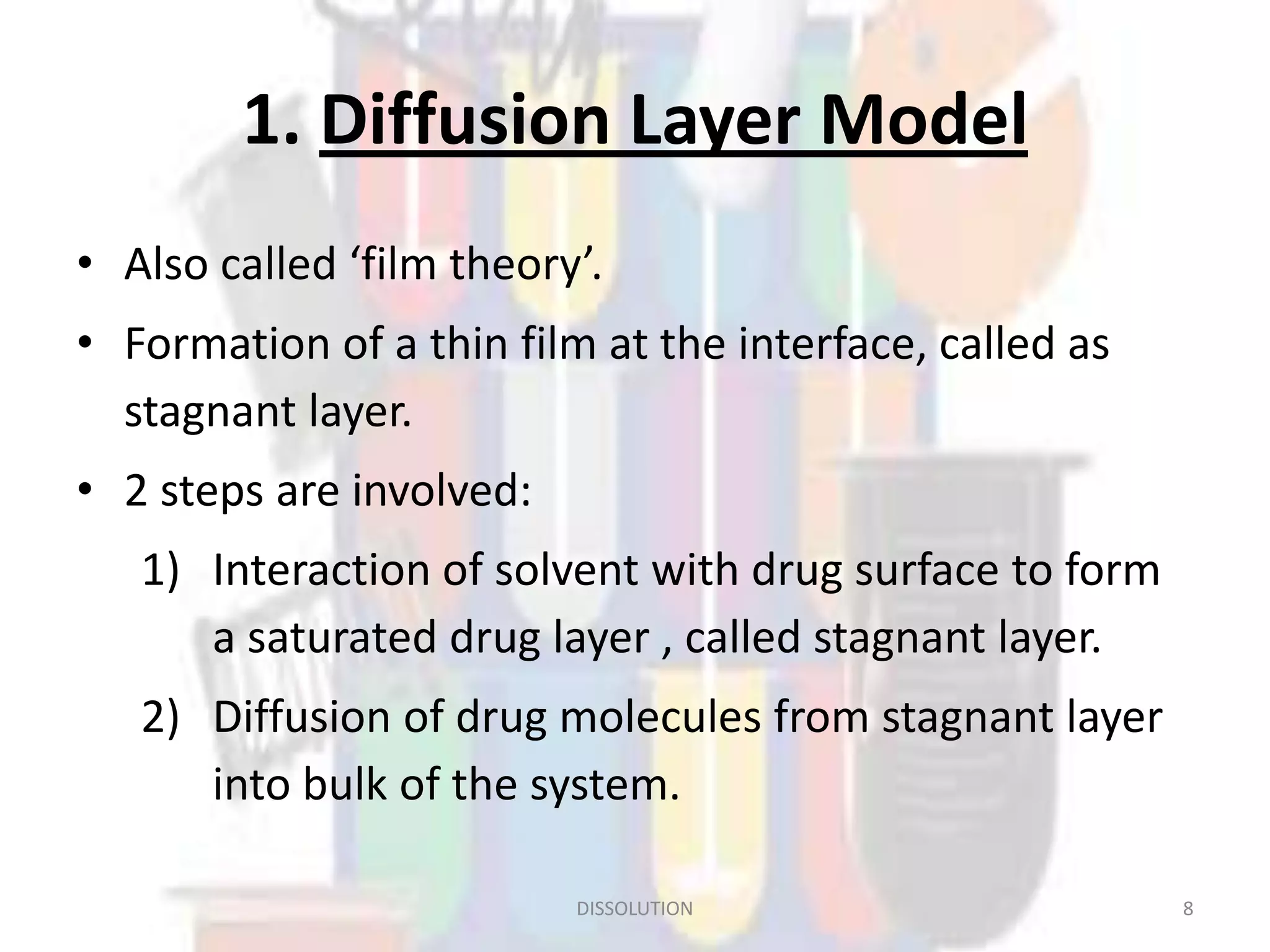
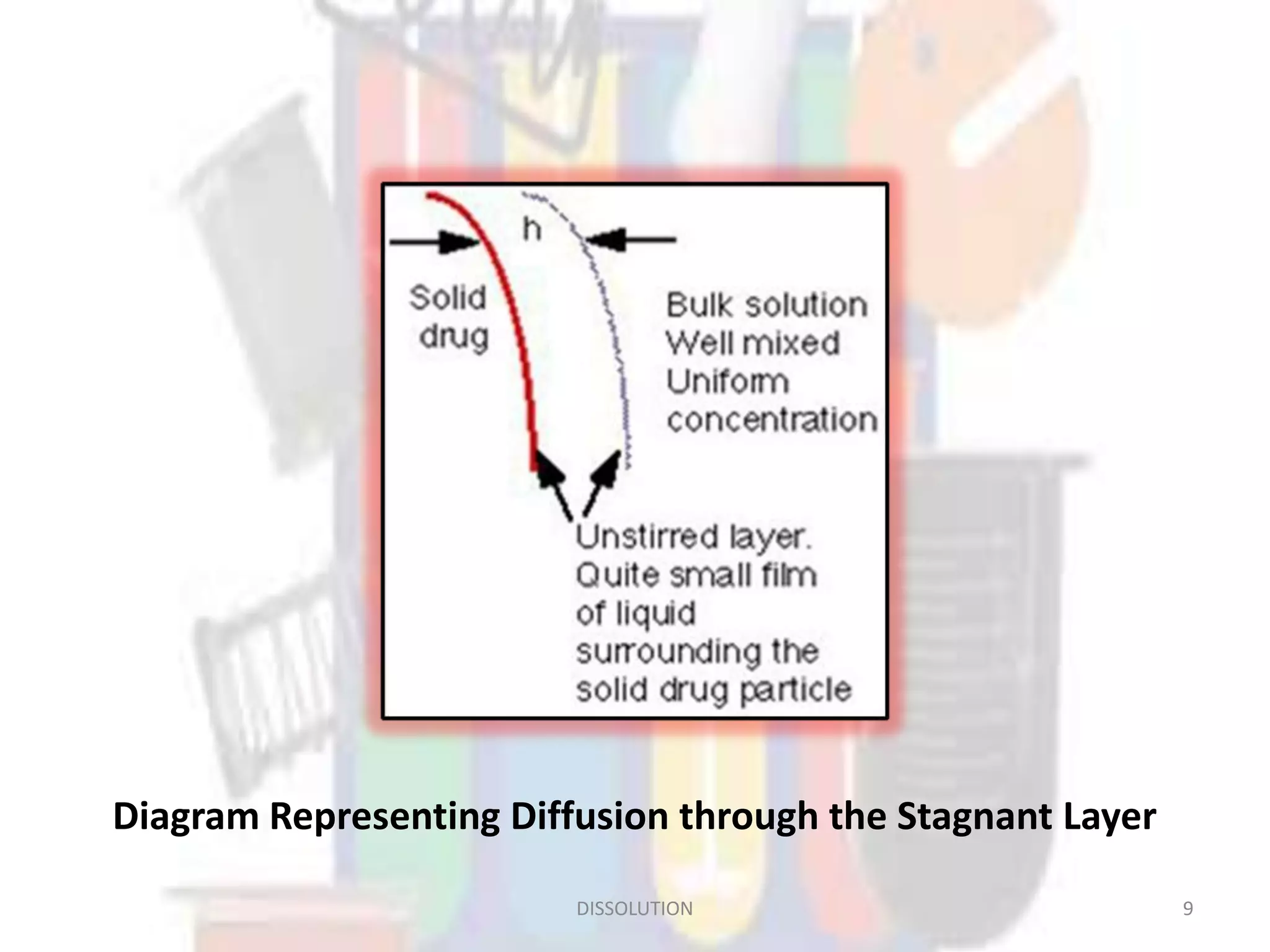
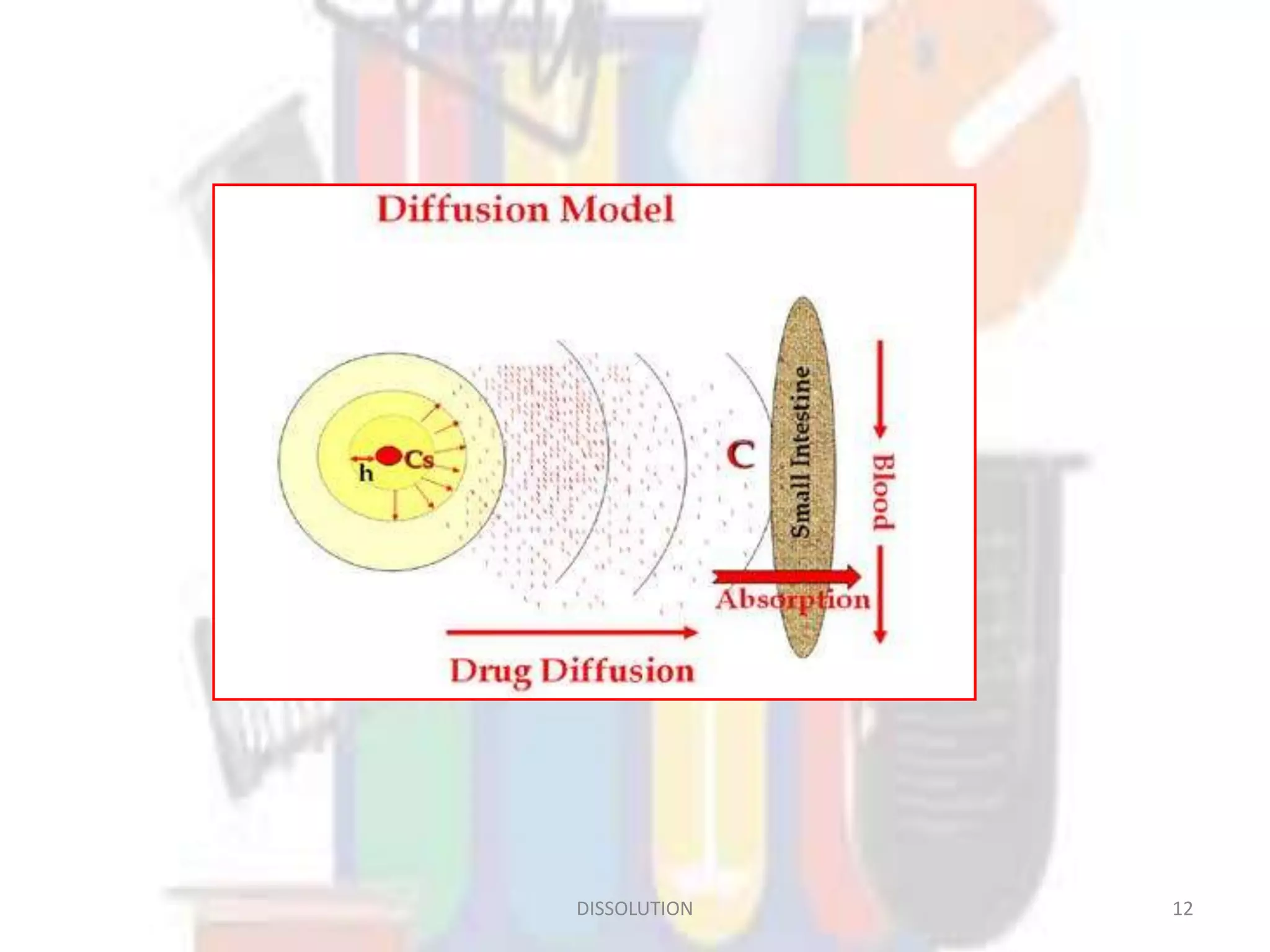
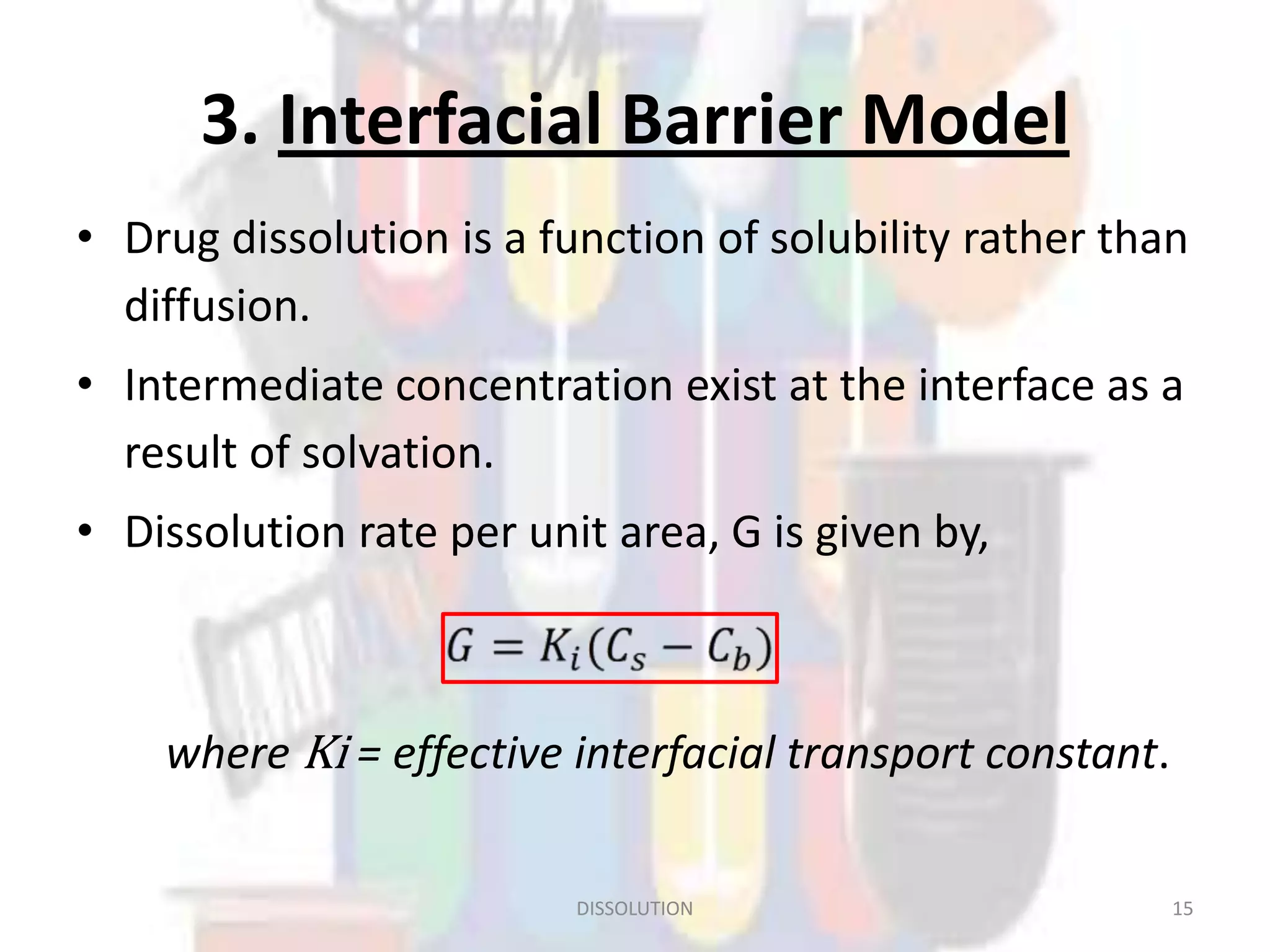
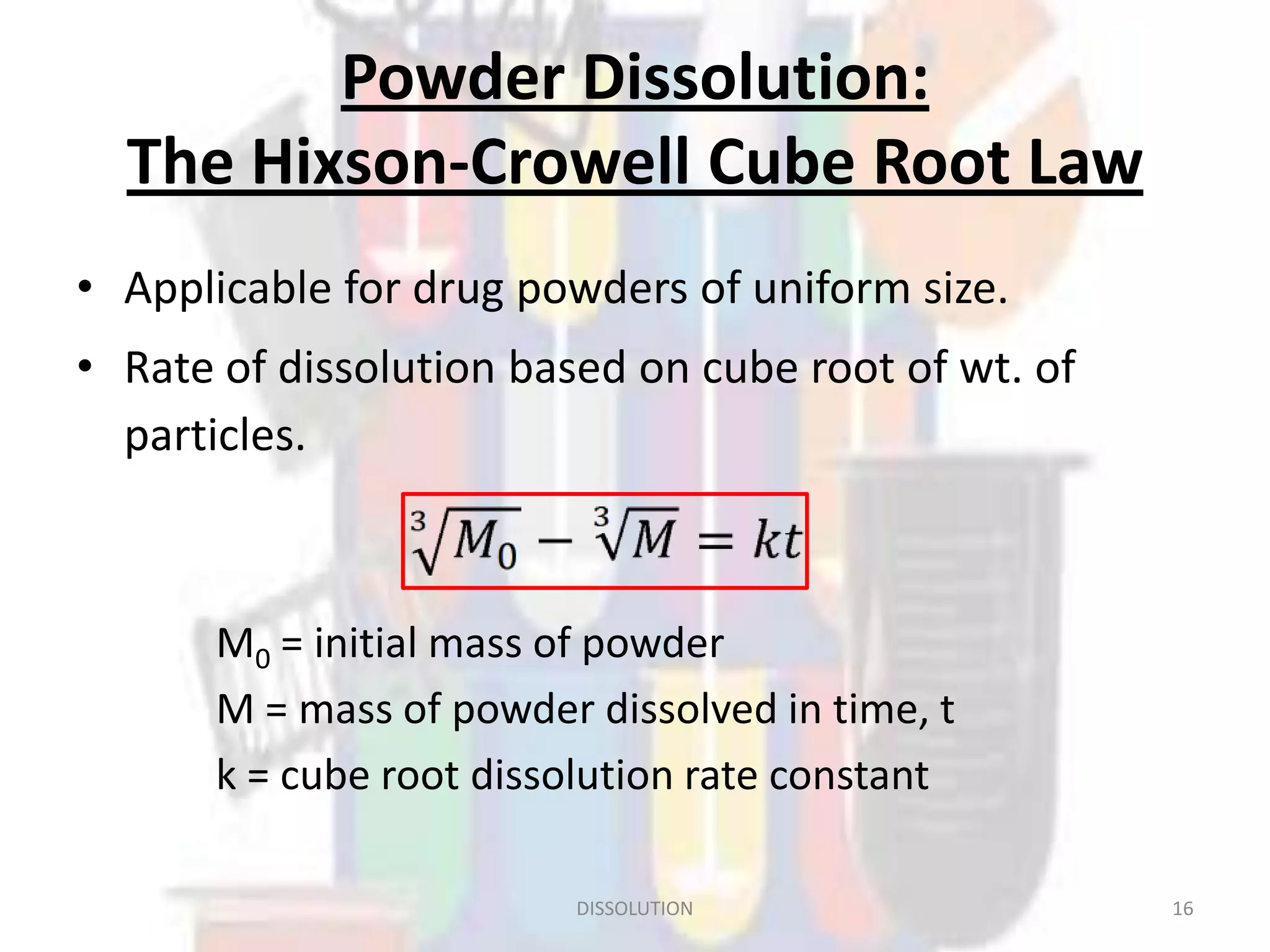
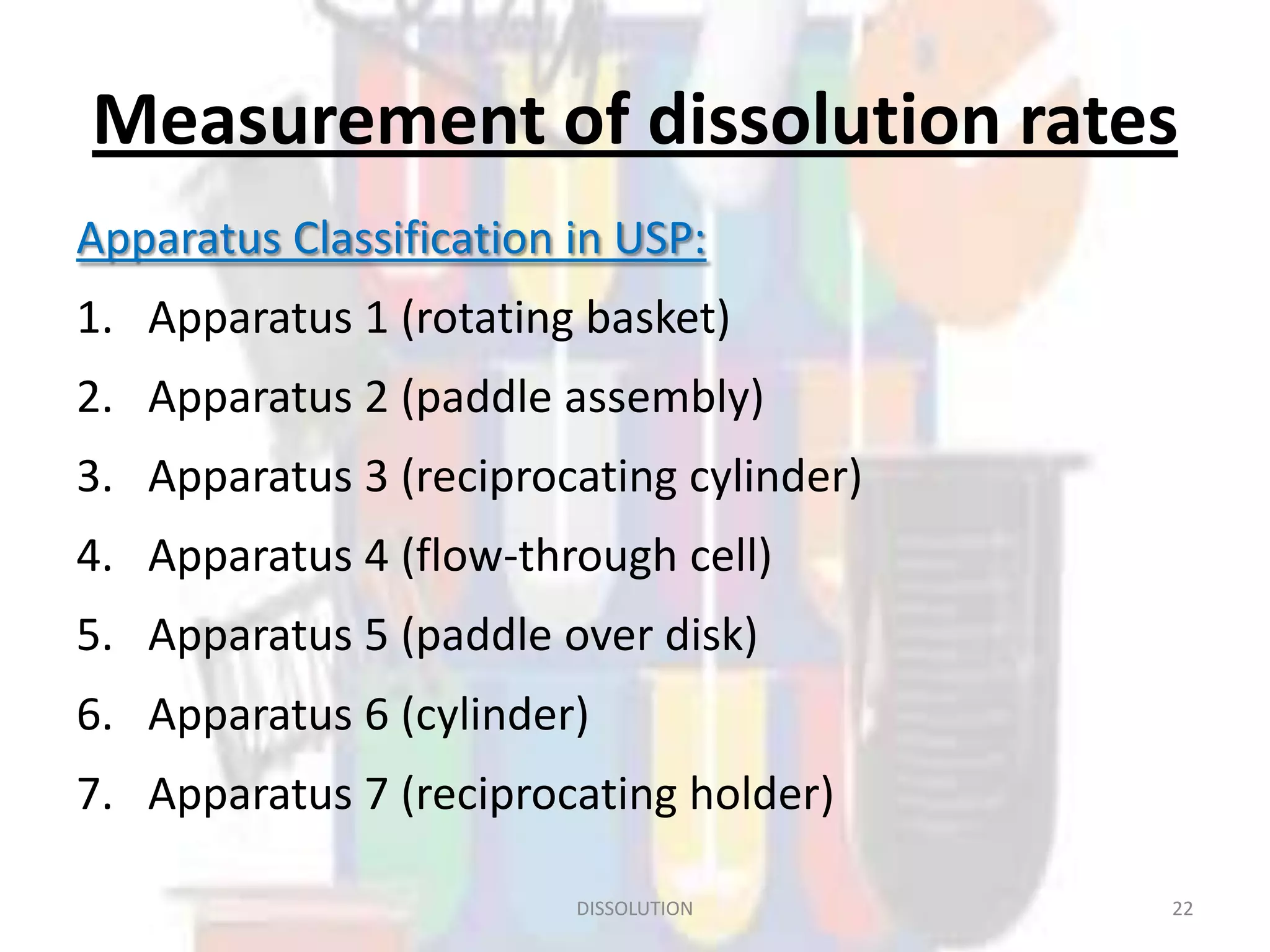
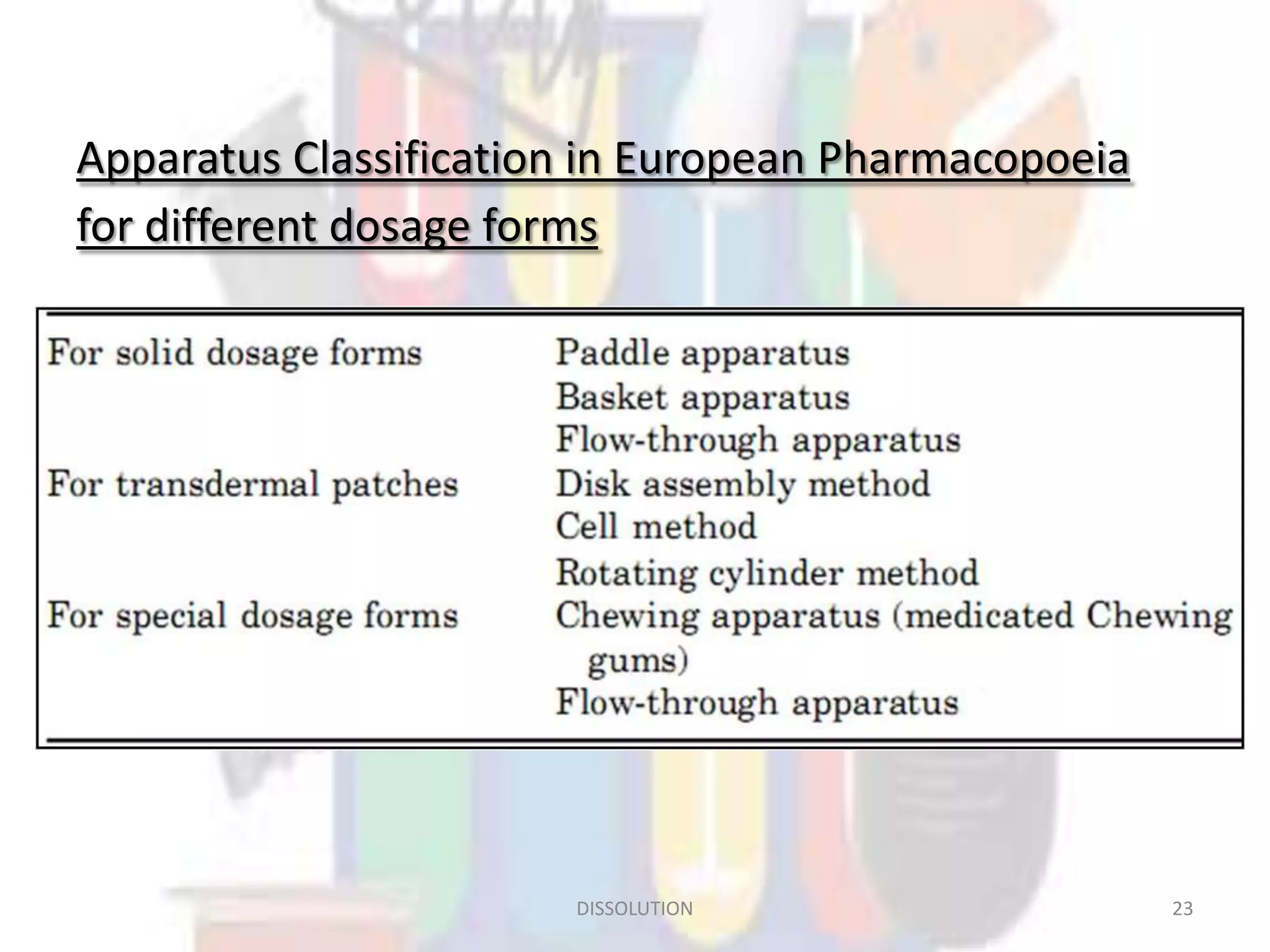
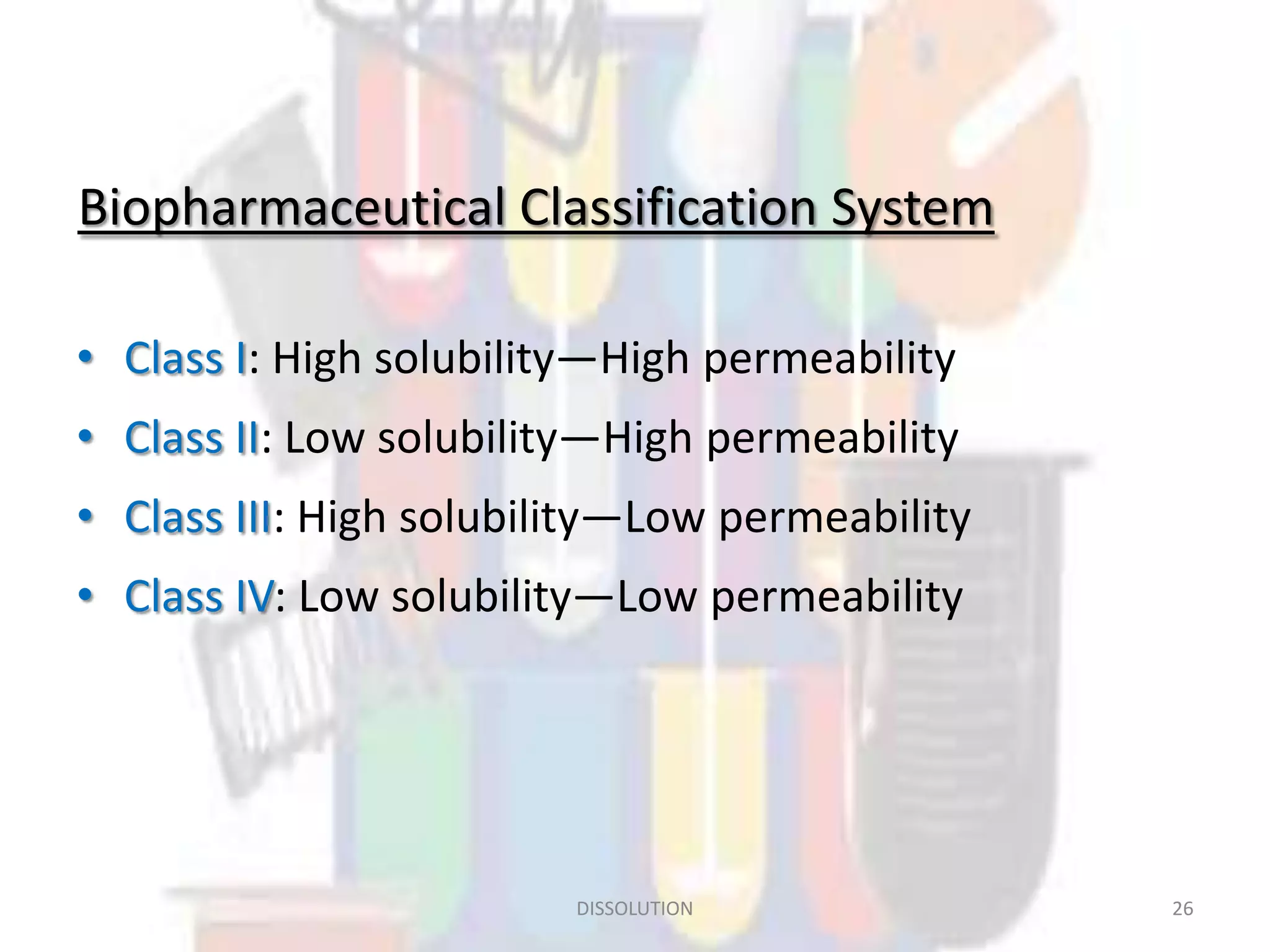
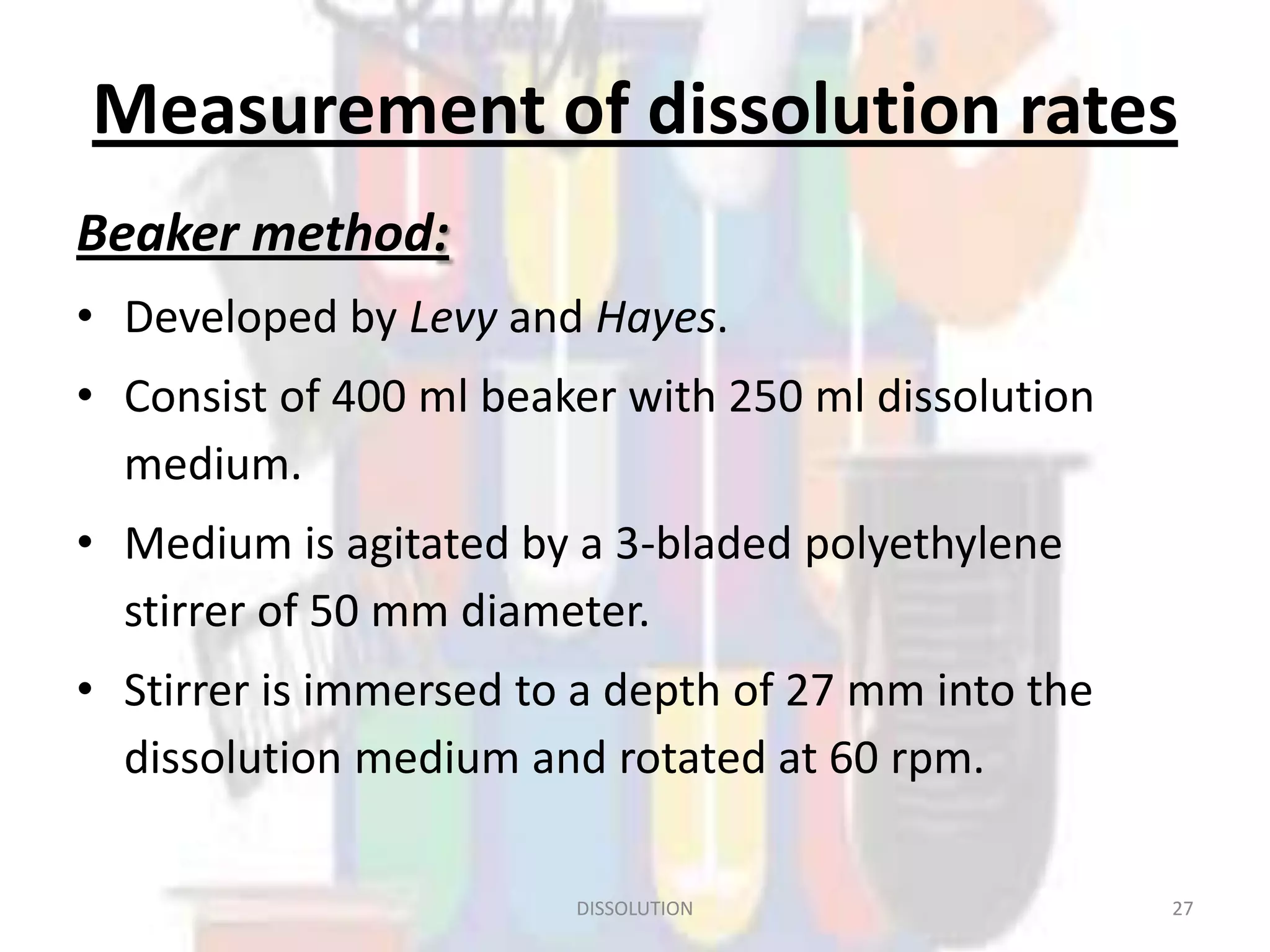
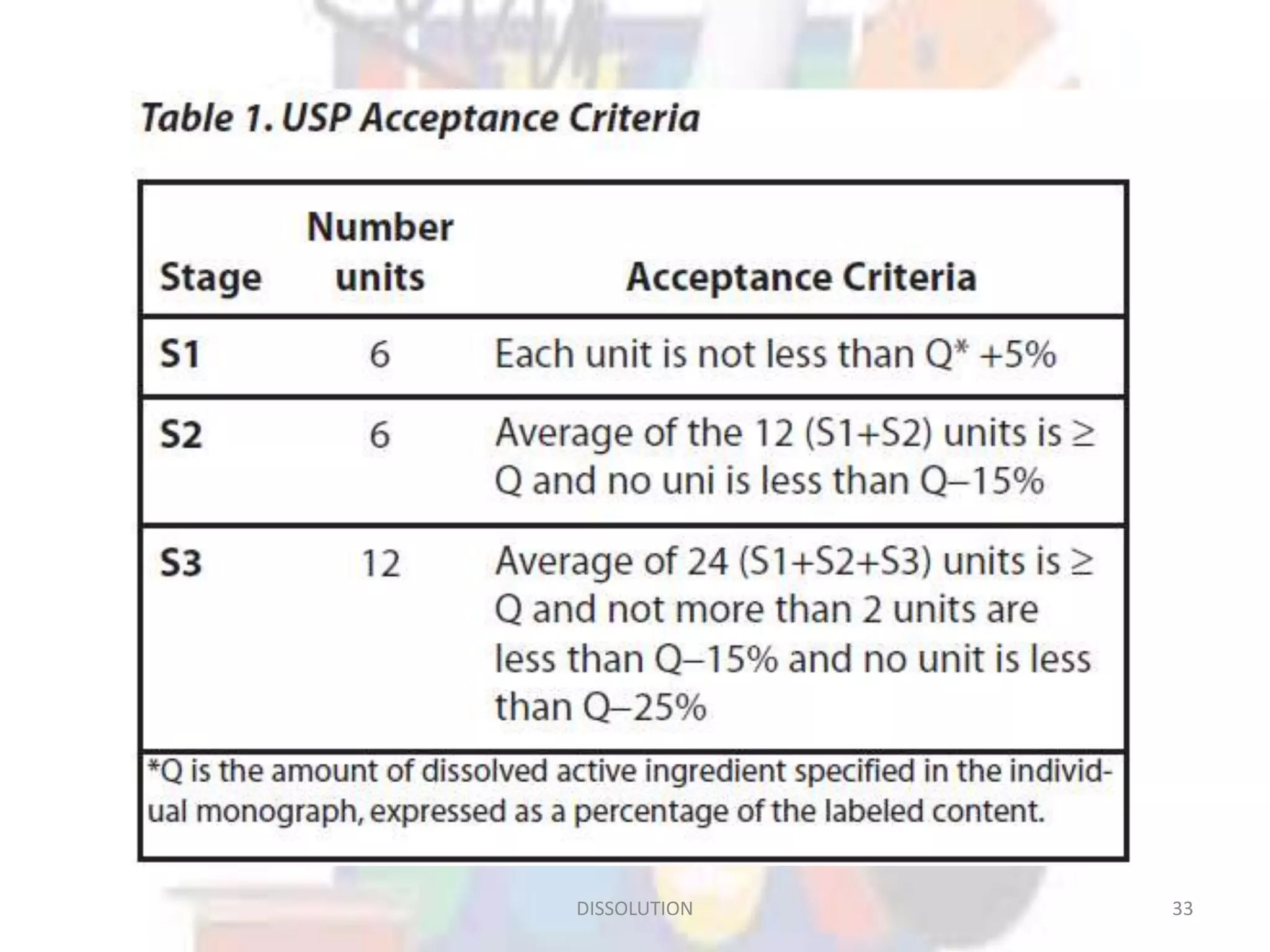
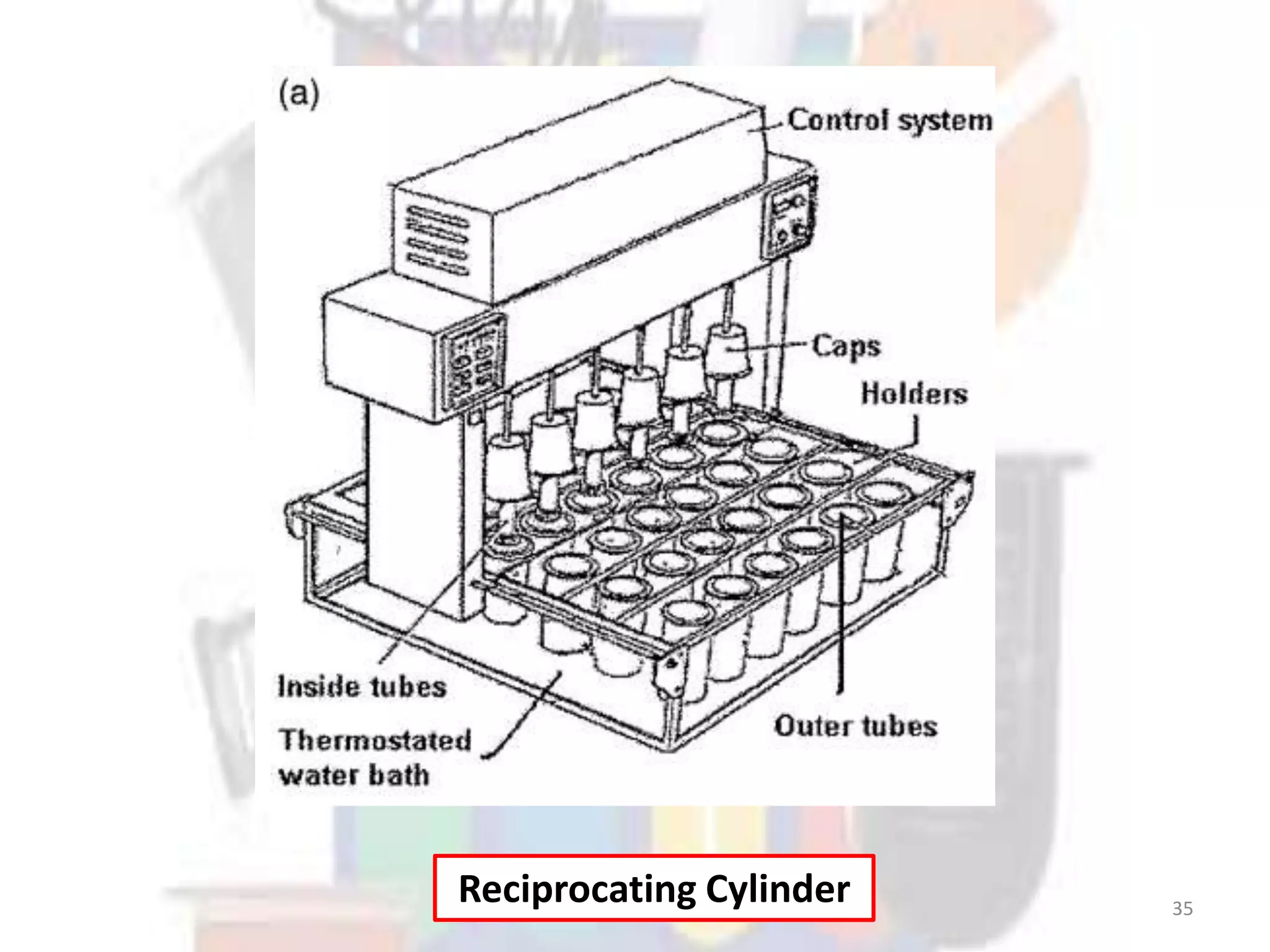
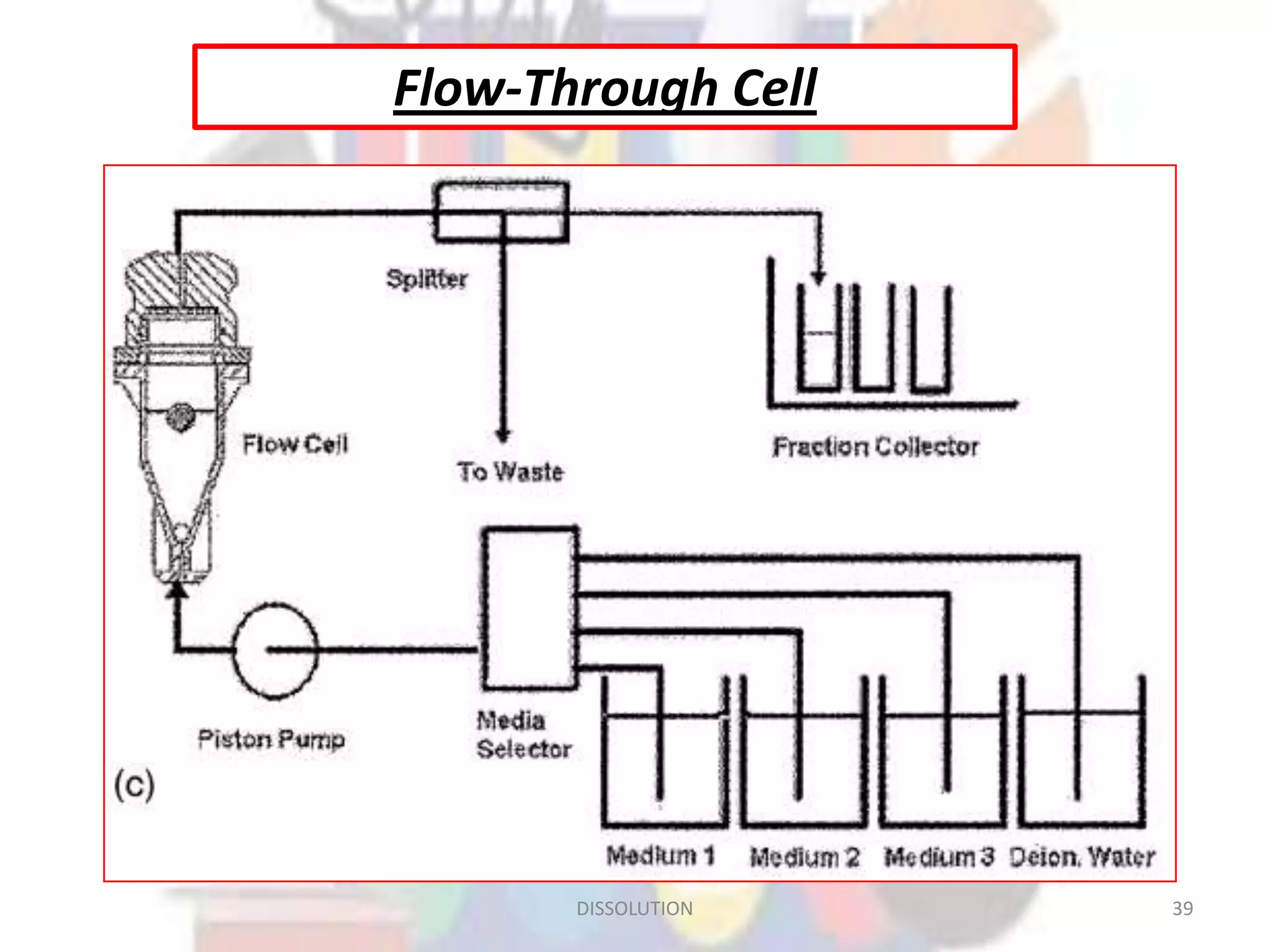
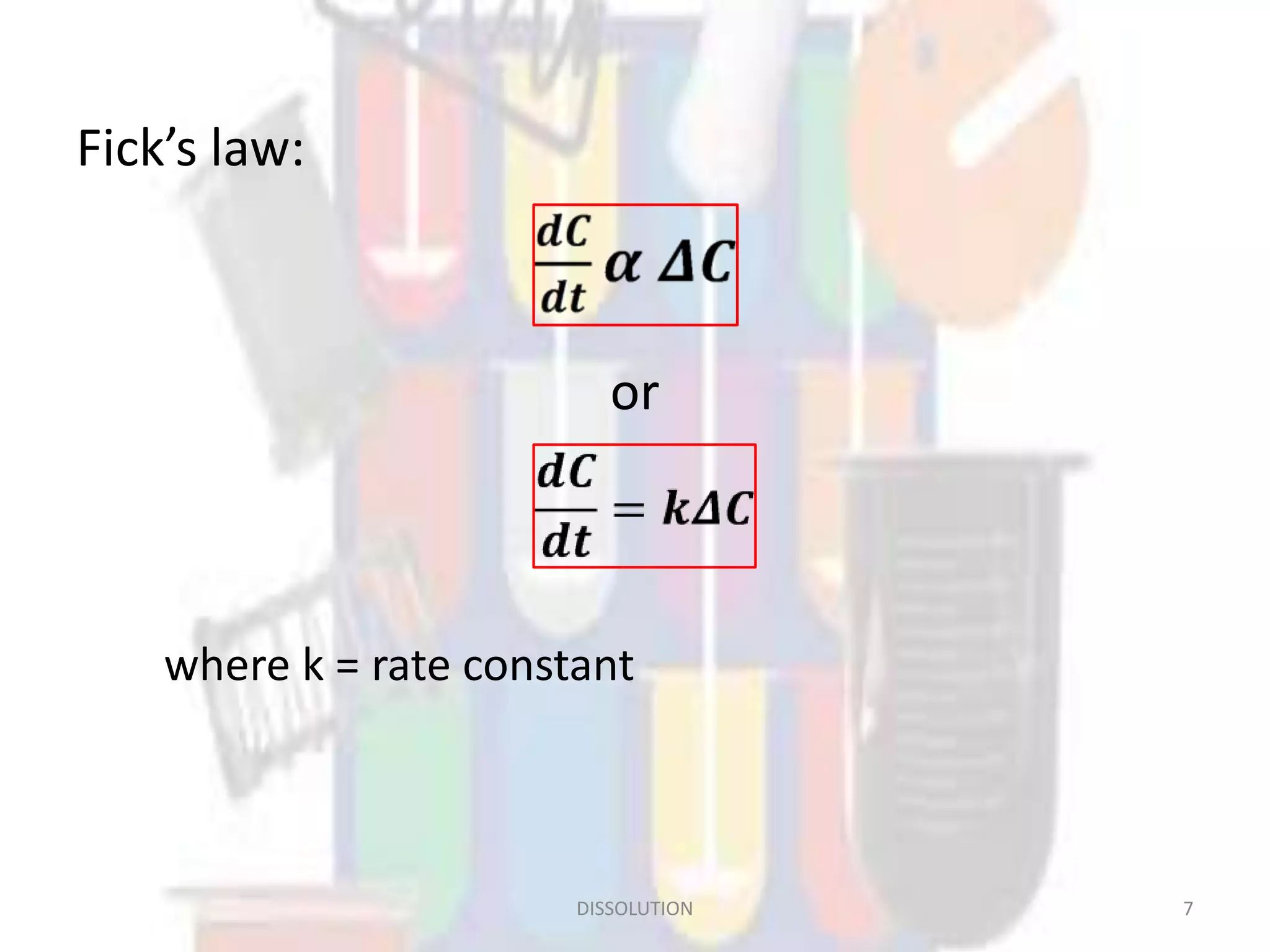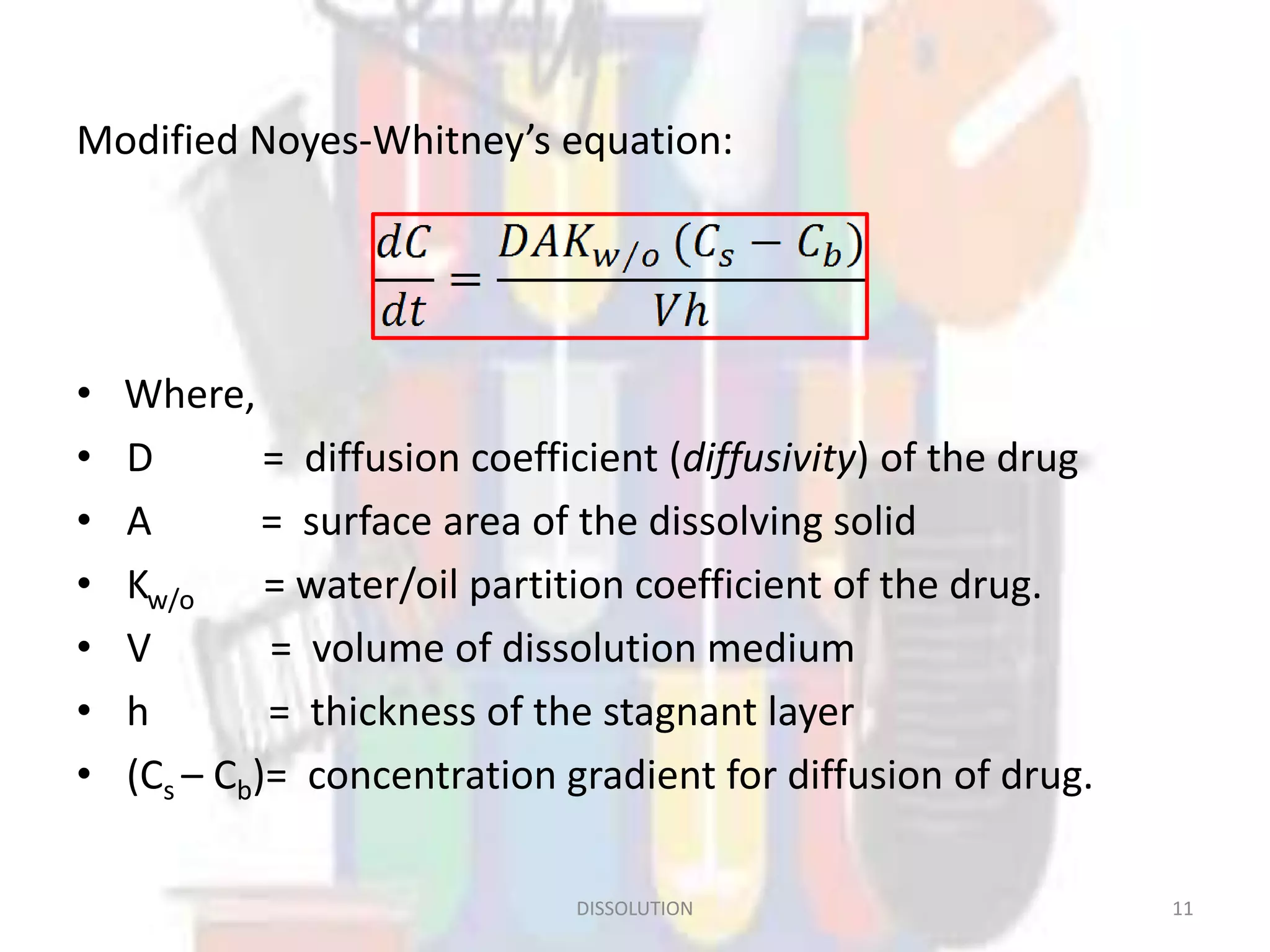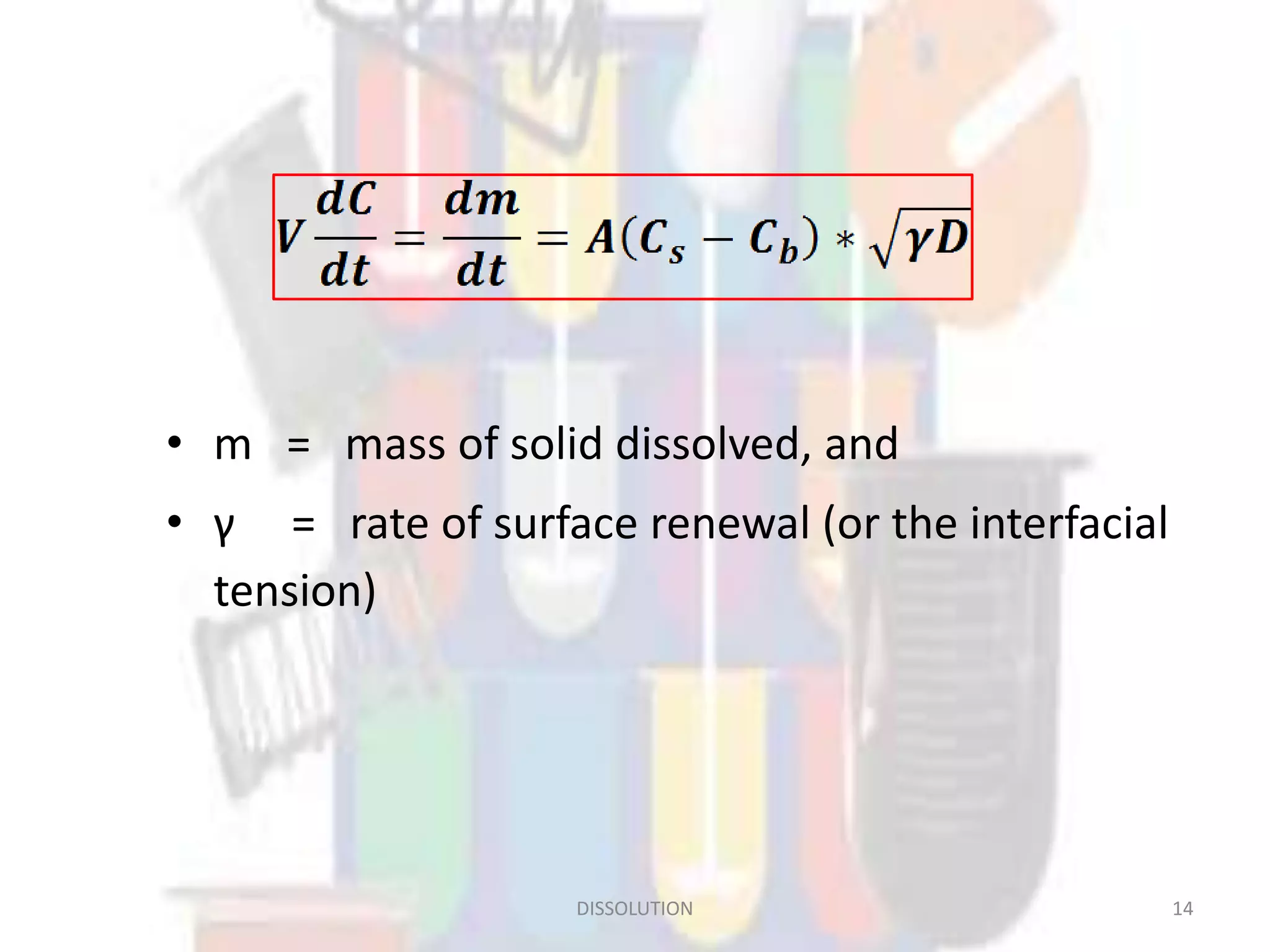Dissolution refers to the process where a solid substance solubilizes in a solvent, which is crucial for the release of poorly water-soluble drugs. Different models describe the mechanisms of dissolution, including the diffusion layer model and interfacial barrier model, with the overall rate determined by the slowest step. Various methods and apparatus are used for testing dissolution rates, with defined criteria for rapid dissolving products according to pharmacopoeial standards.