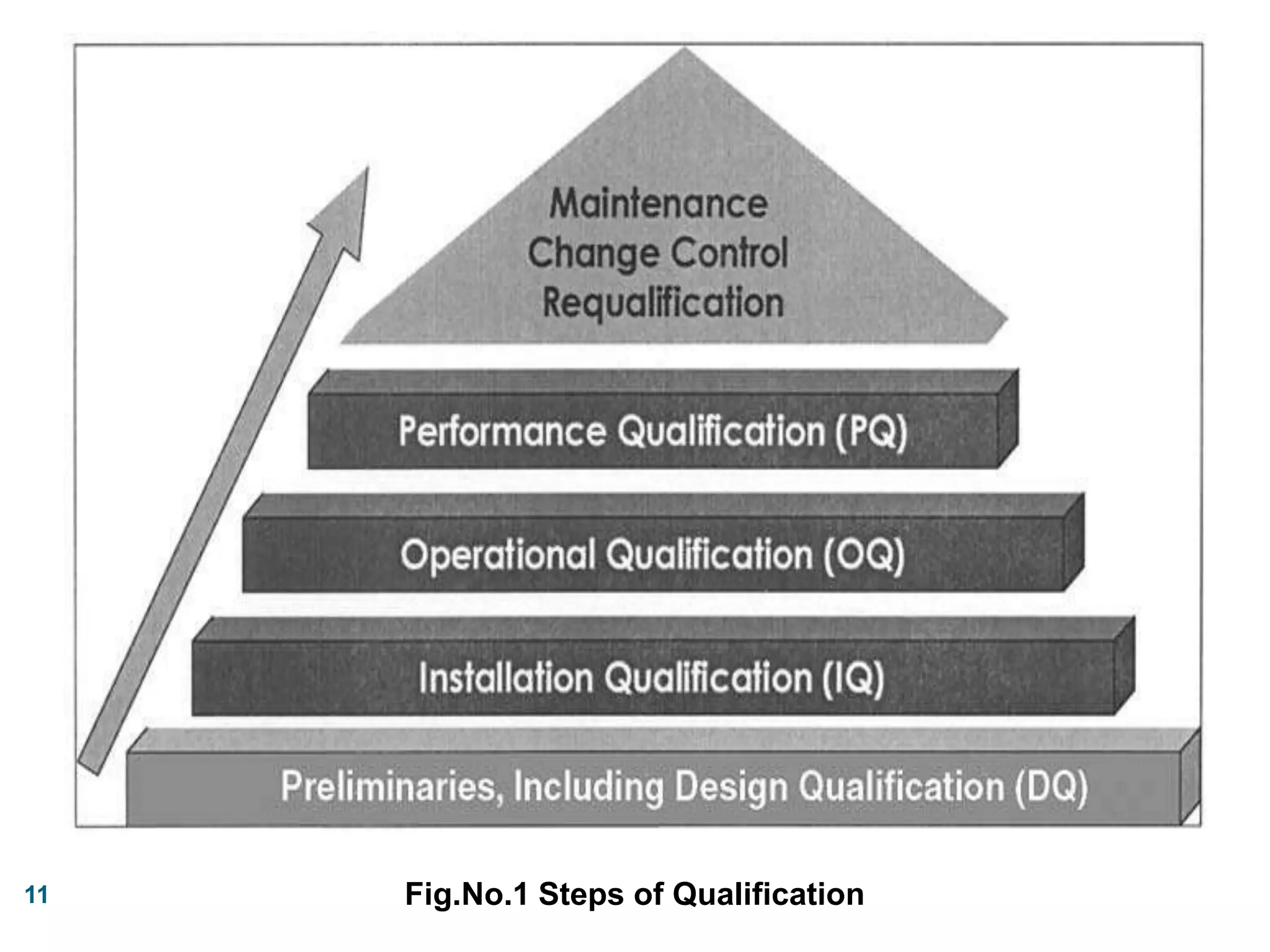
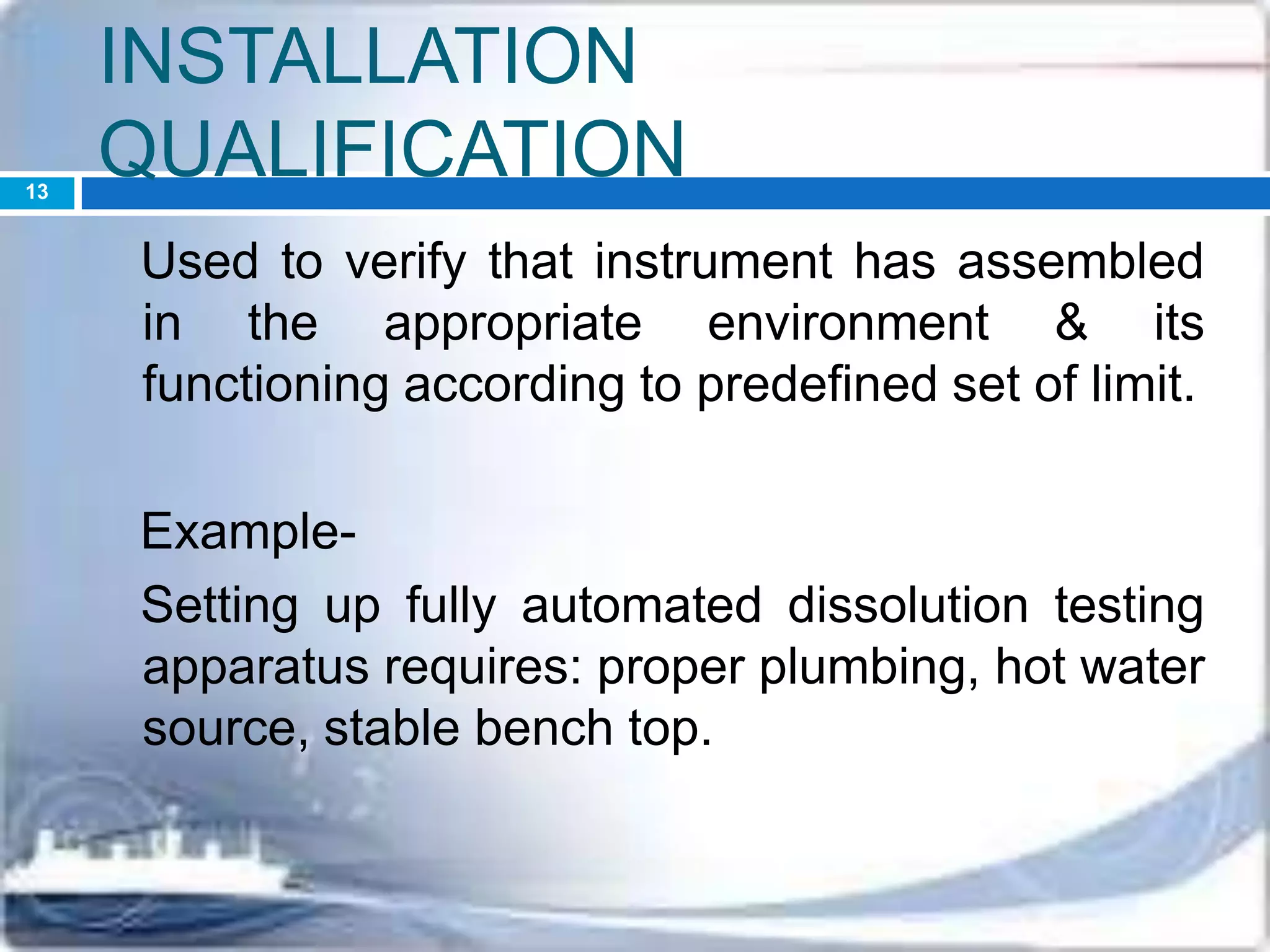
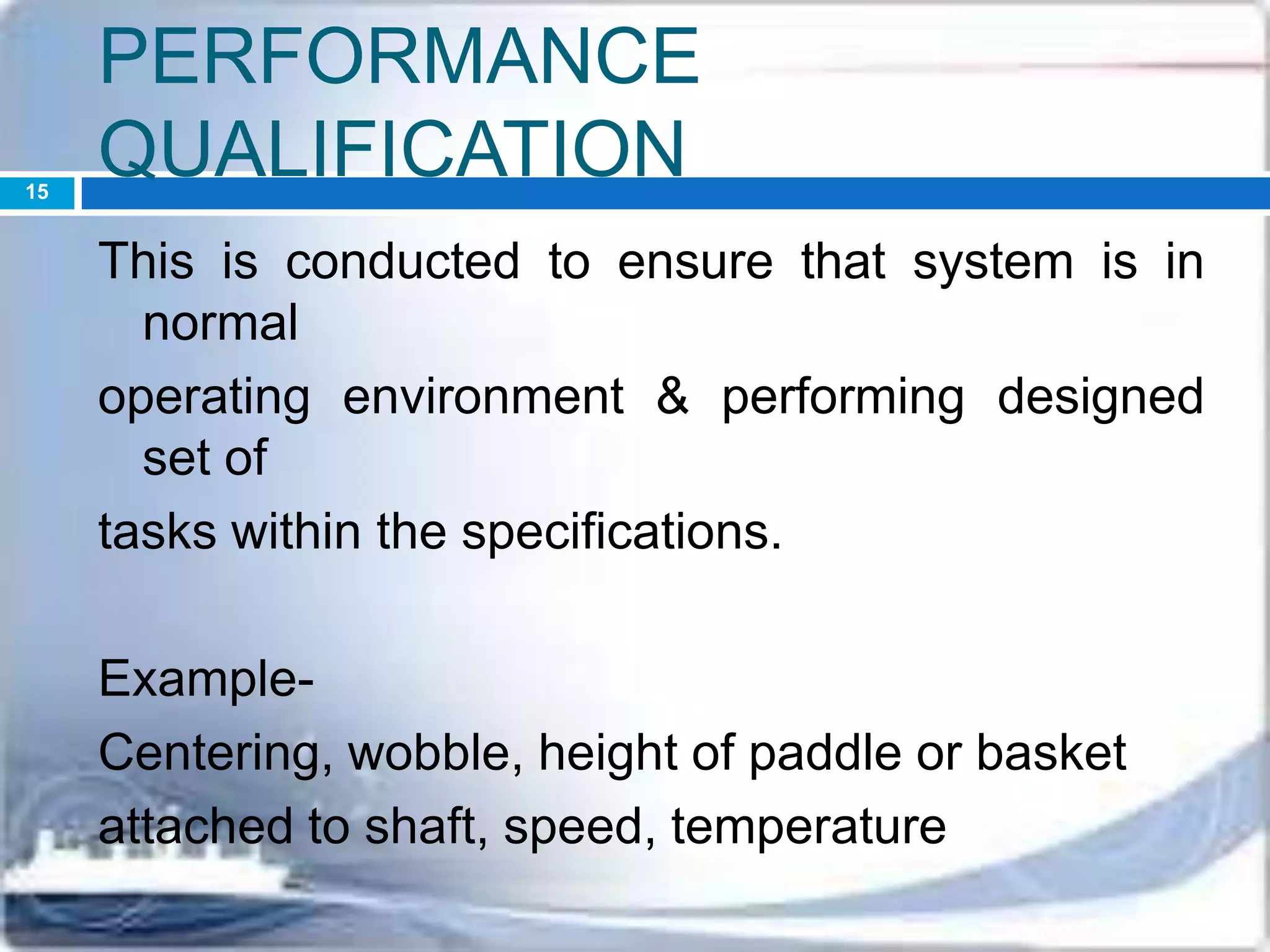
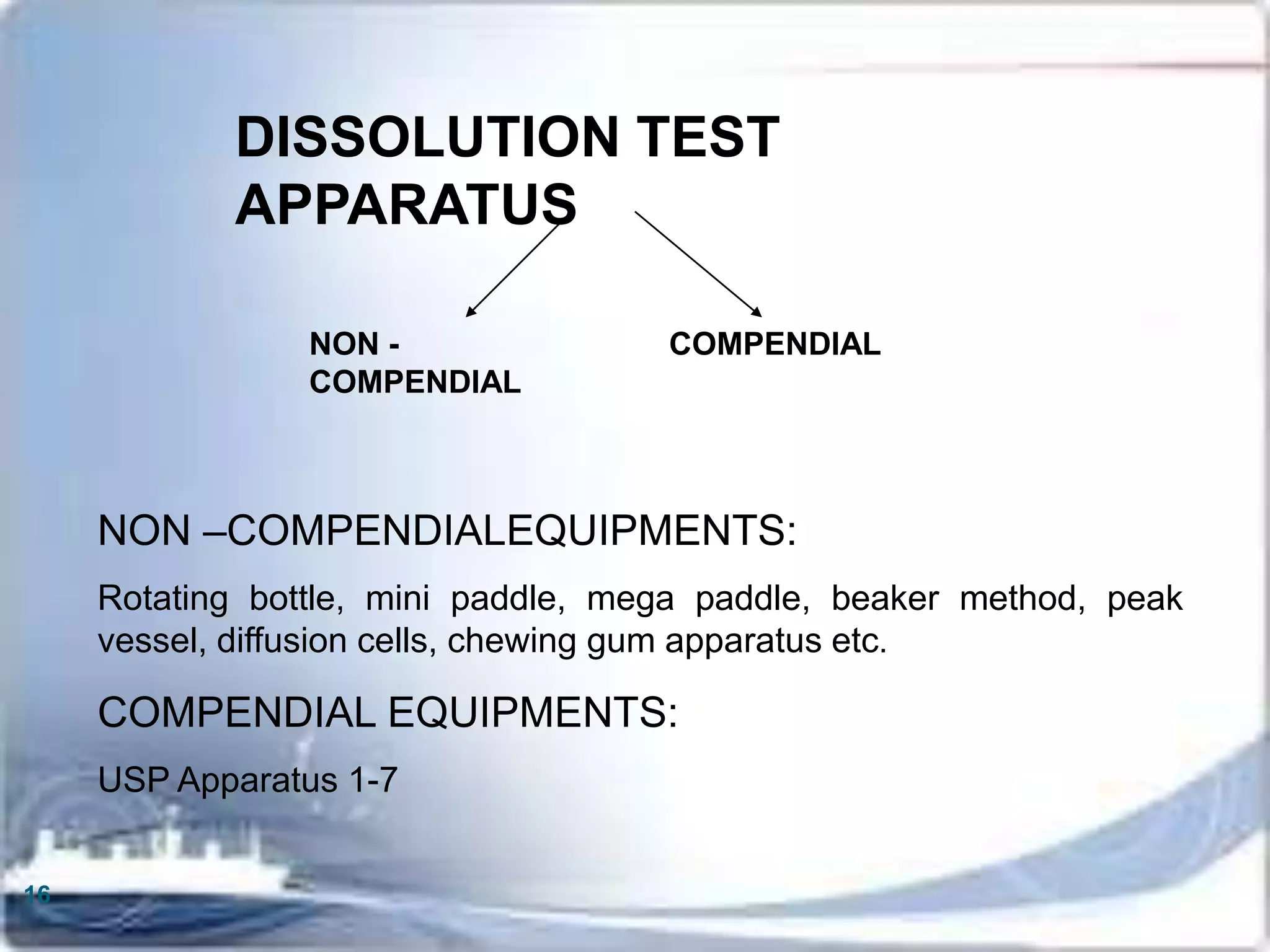
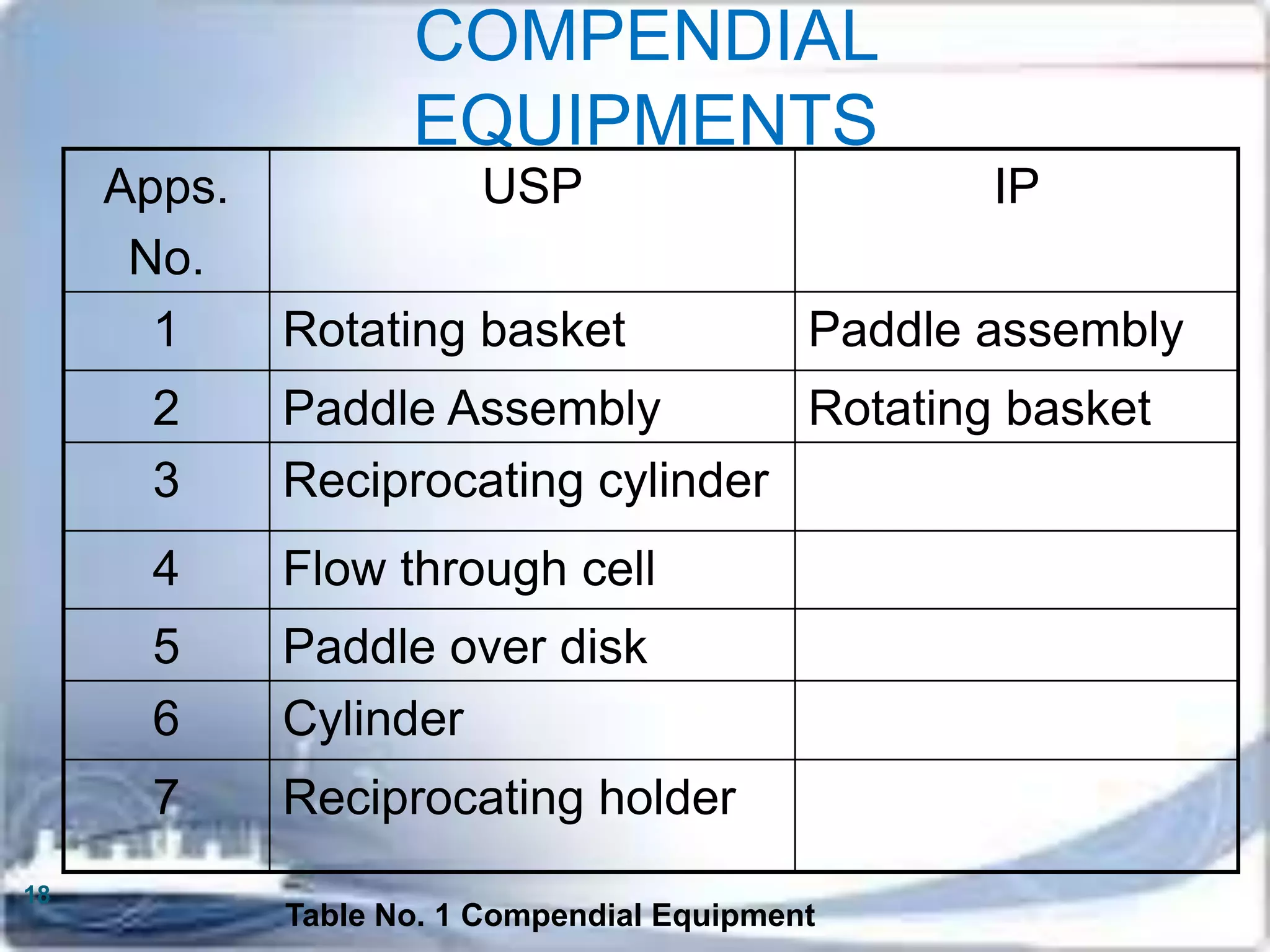
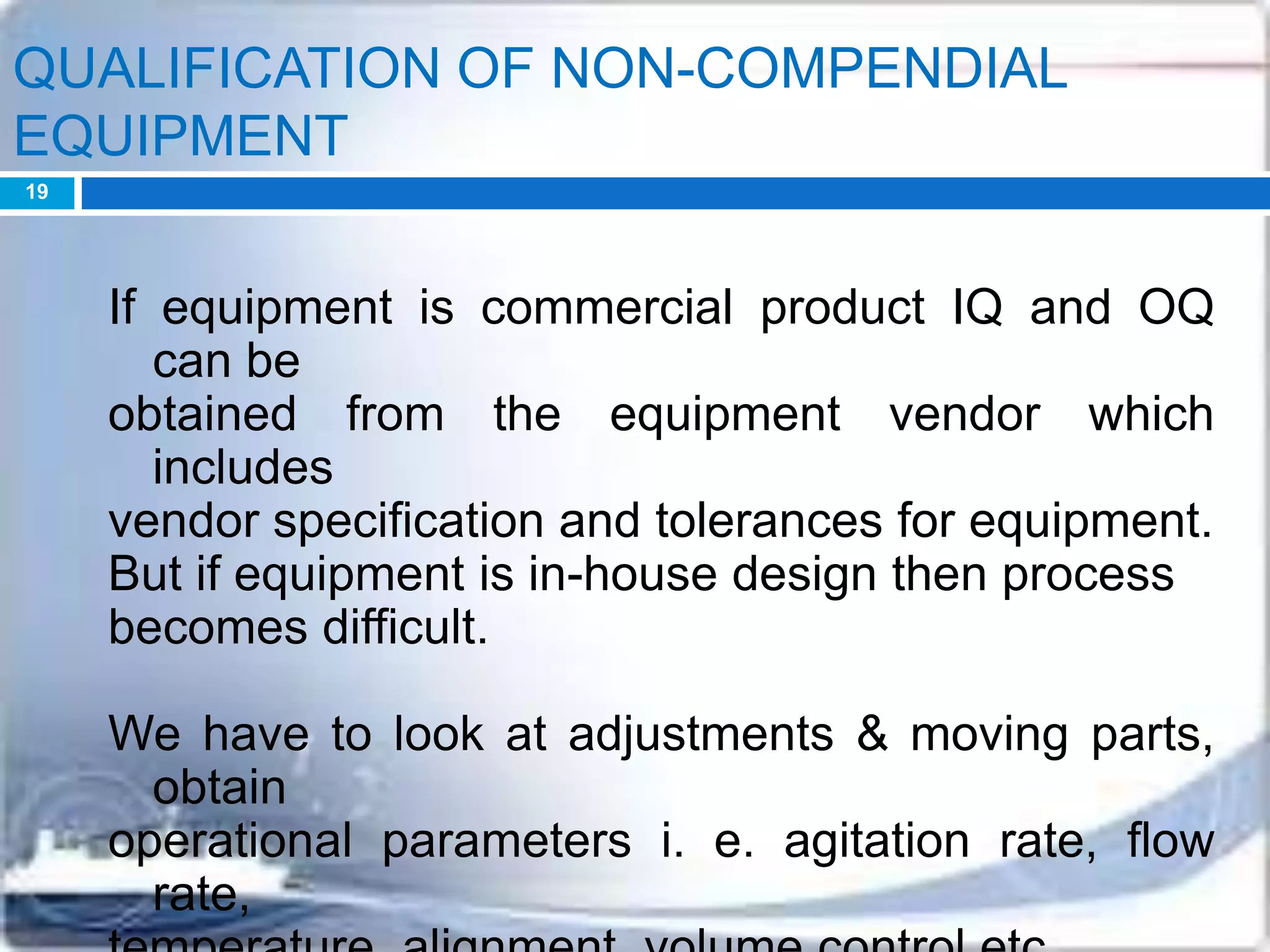
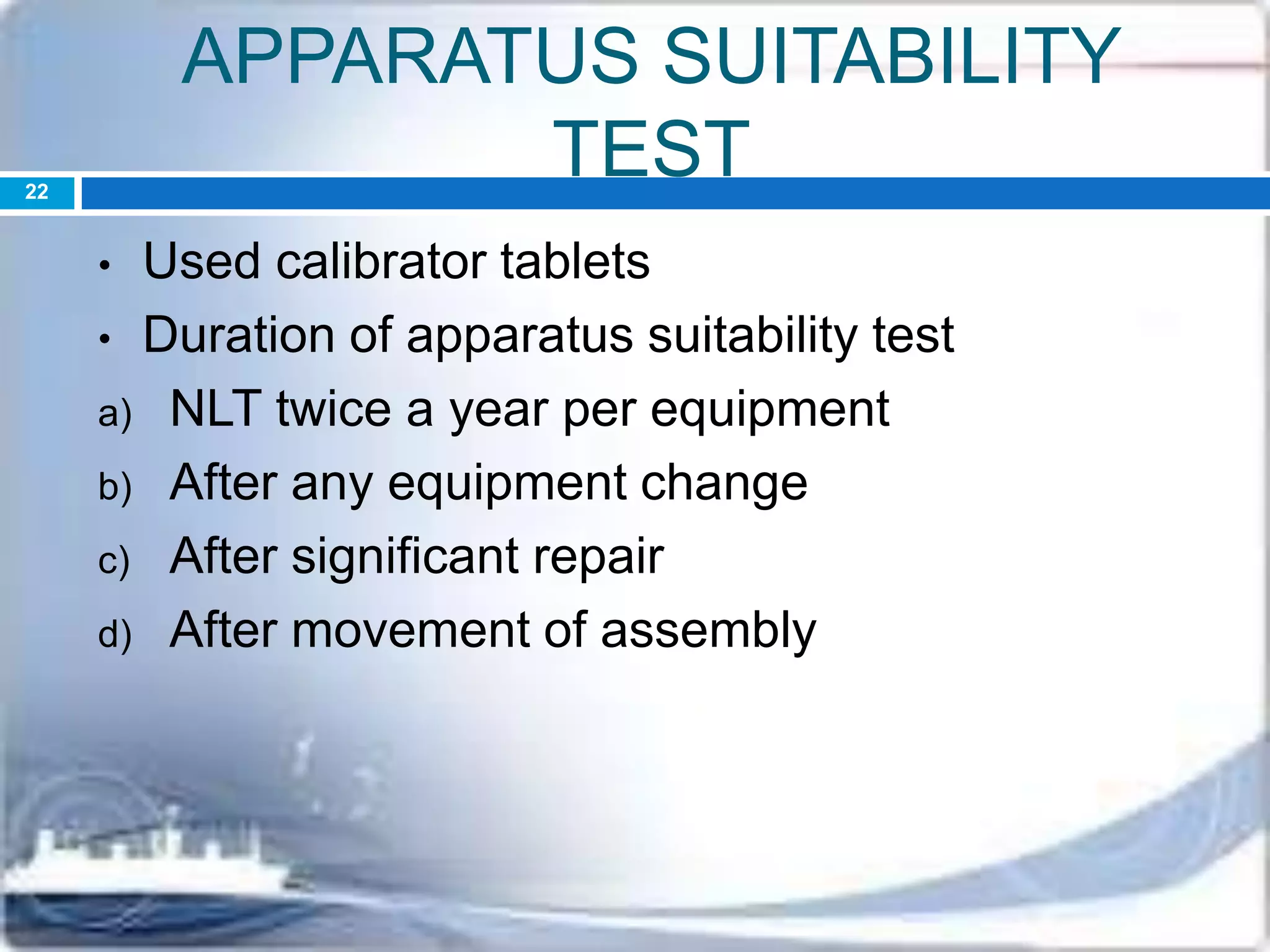
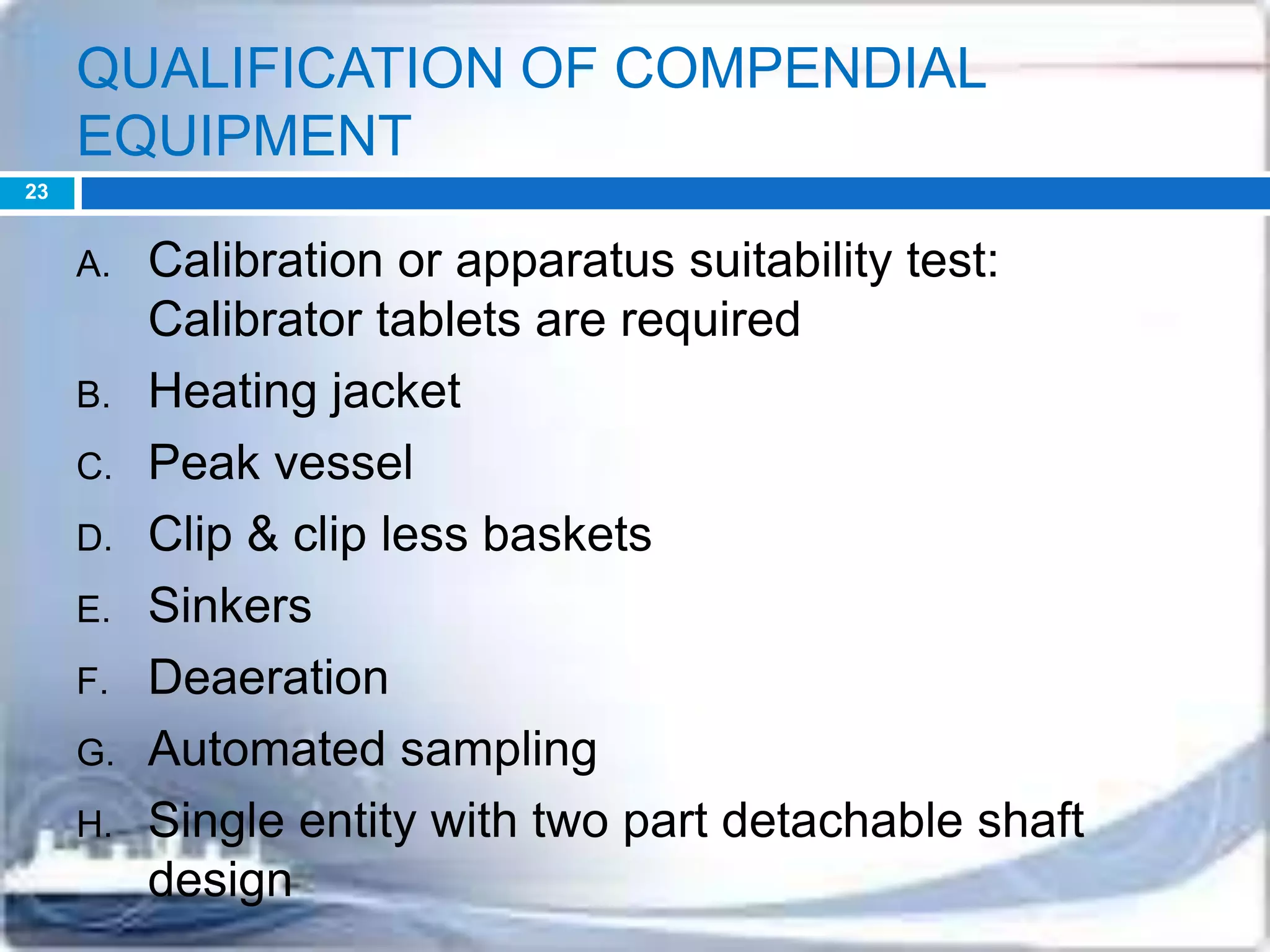
This document discusses the validation of dissolution test apparatus. It begins with a brief history of validation and reasons for validating equipment. Validation ensures equipment operates consistently and accurately. The document then discusses various types of dissolution test apparatus and the qualification process, including design, installation, operational, and performance qualification. It also addresses sources of error and concludes that acceptable qualification demonstrates the apparatus is validated for use in dissolution testing.











![METHODS
A] Calibration
Non-compendial & some compendial apparatus
do not have calibrator tablets, in some case in
house calibrator tablets are designed.
Some unique aspects of equipment can only be
detected with USP calibrated tablets, there are
no practical measuring tools available for
analyst. e.g. vibration and vessel
irregularities.
20](https://image.slidesharecdn.com/validationofdissolutionapparatus-140409075234-phpapp01/75/Validation-of-dissolution-apparatus-20-2048.jpg)
![B] HYDRODYNAMICS
Dissolution fluid flow should be free from
irregularities
or variable turbulence.
Highly variable data may be unsuitable for that
product.
C] OTHER CONSIDERATIONS-
Ruggedness should be thoroughly evaluated
before
considering transferring product testing to
another site.21](https://image.slidesharecdn.com/validationofdissolutionapparatus-140409075234-phpapp01/75/Validation-of-dissolution-apparatus-21-2048.jpg)

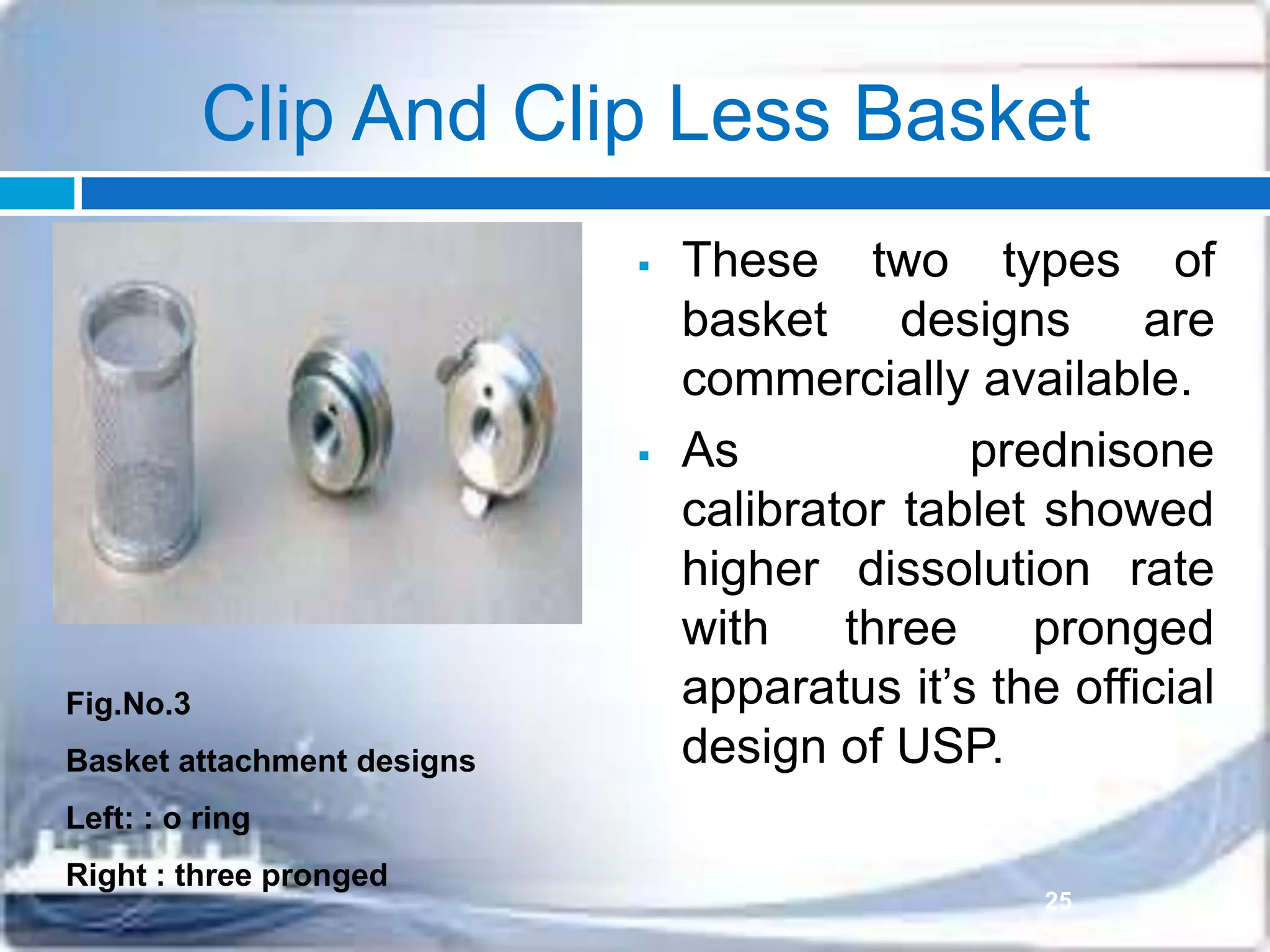



![SOURCES OF ERRORS
A] Drug substance properties
e.g. Solubility, pH
B] Drug product properties
Mechanical, formulation
C] Equipment
Mechanical & chemical aspects, Apparatus suitability test
D] Method consideration
To avoid errors
Film coated tablets-sticky-sinker
Suspension-syringe/pipette/beaker
Medium-volume difference
Presence of surfactants
33](https://image.slidesharecdn.com/validationofdissolutionapparatus-140409075234-phpapp01/75/Validation-of-dissolution-apparatus-33-2048.jpg)
![SOURCES OF ERRORS
E] Observation
Sinkers-turns of wire helix-its effect
Manual sampling
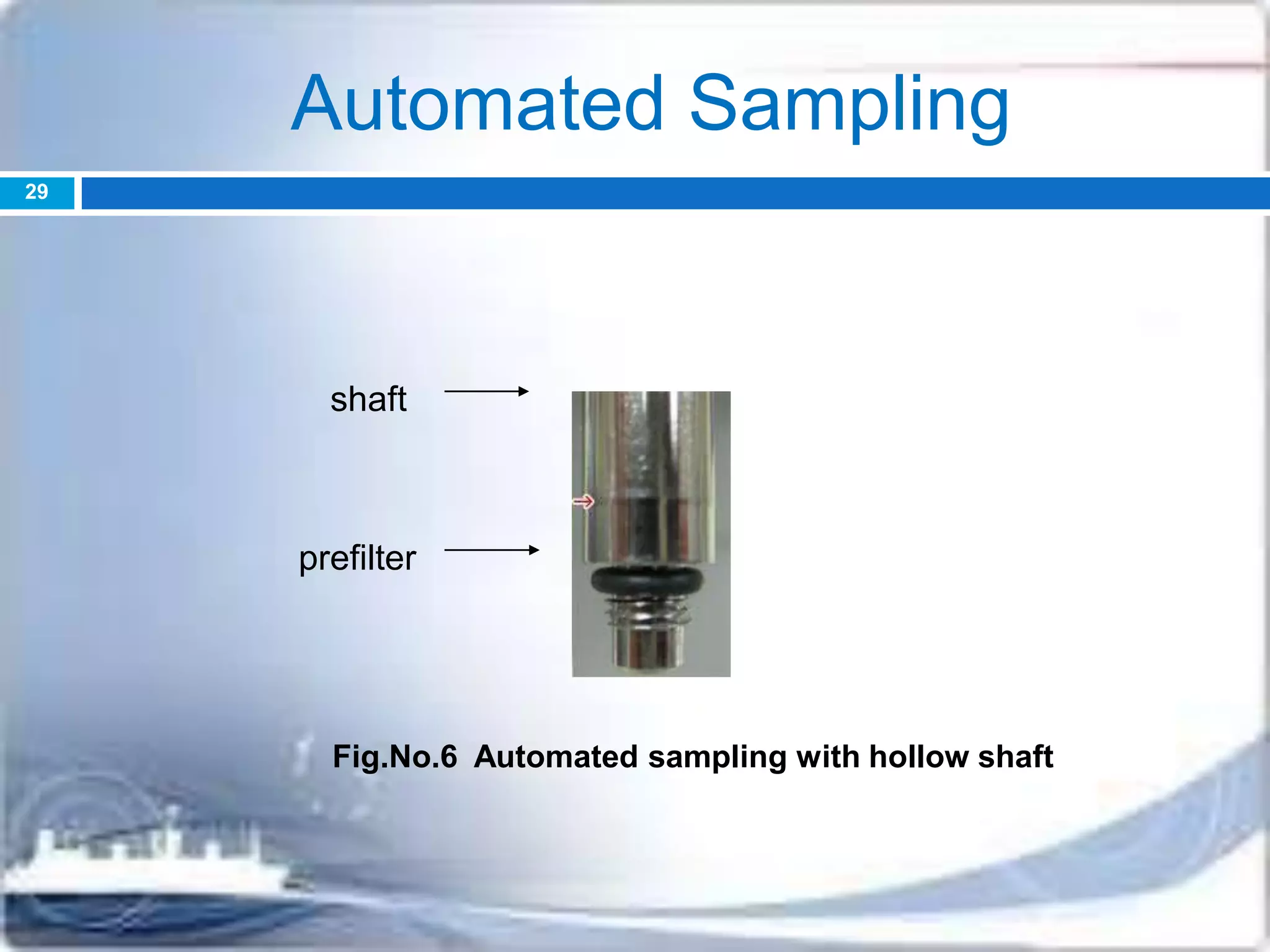
F] Automation
Problems-disconnection, inadequate cleaning, mix-ups
G] Cleaning
Many products, same equipment major source of error
H] Method transfer
Sinkers, dispensing apparatus, sampling methods, precise
medium, standard preparations, grade of reagents
should be uniform.
34](https://image.slidesharecdn.com/validationofdissolutionapparatus-140409075234-phpapp01/75/Validation-of-dissolution-apparatus-34-2048.jpg)