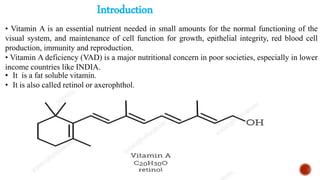
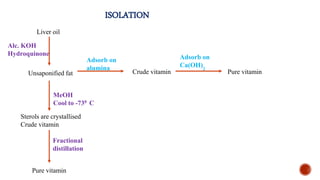
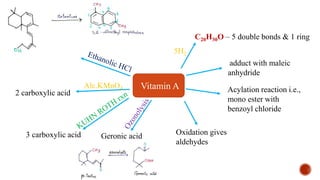
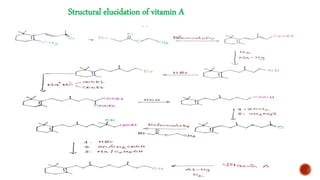
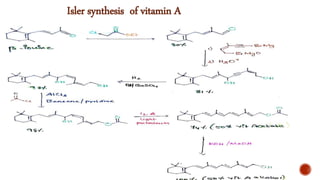
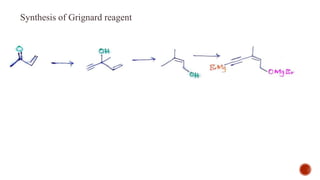
The document provides an overview of Vitamin A, highlighting its importance as a fat-soluble vitamin essential for various bodily functions, including vision and immune support. It covers sources of Vitamin A from both animal and plant origins, its chemical structure, physiological significance, as well as the implications of deficiency and overdose. Additionally, it discusses the history, isolation, and synthesis of Vitamin A, underscoring its critical role in human health.