Embed presentation


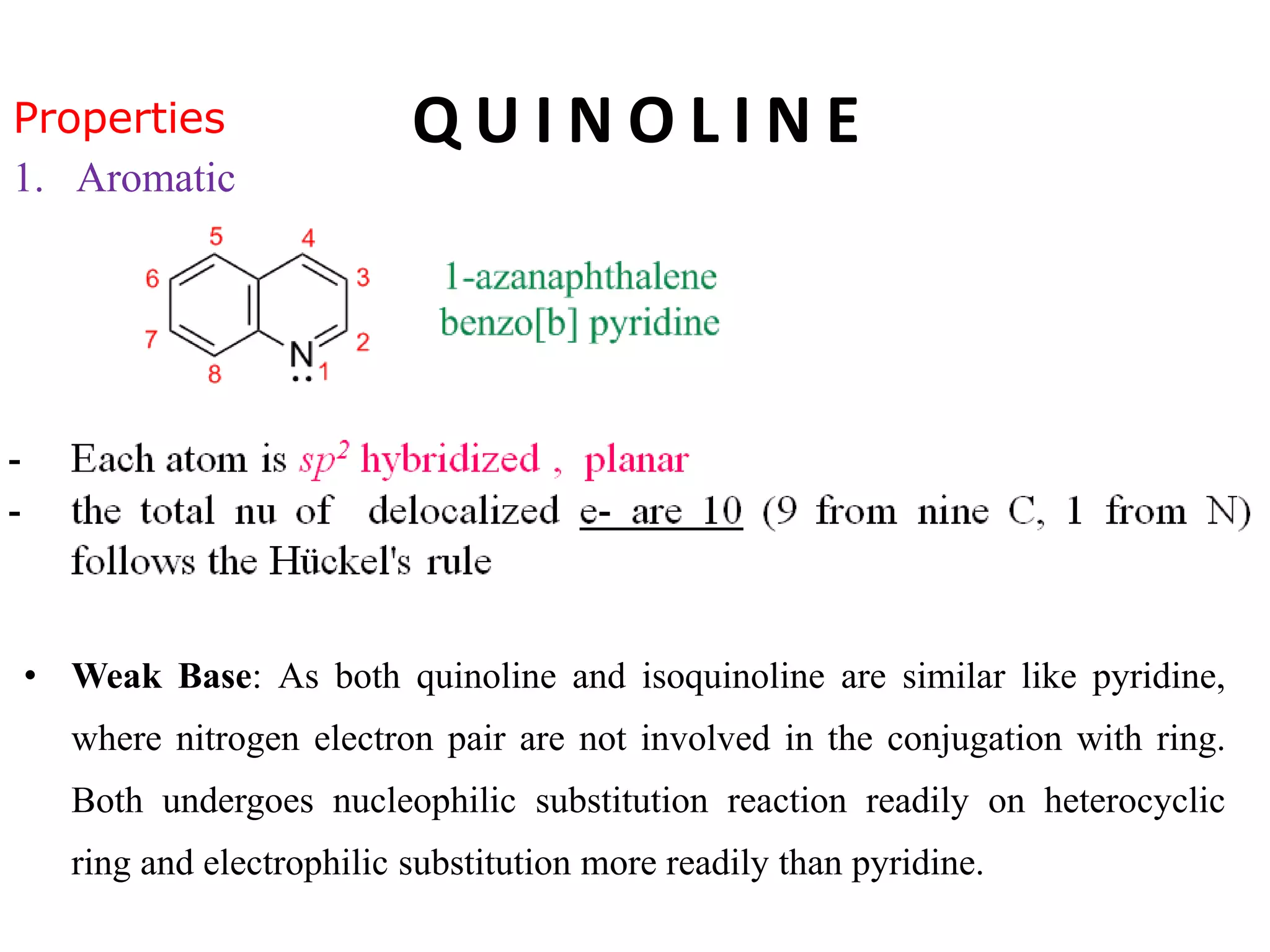
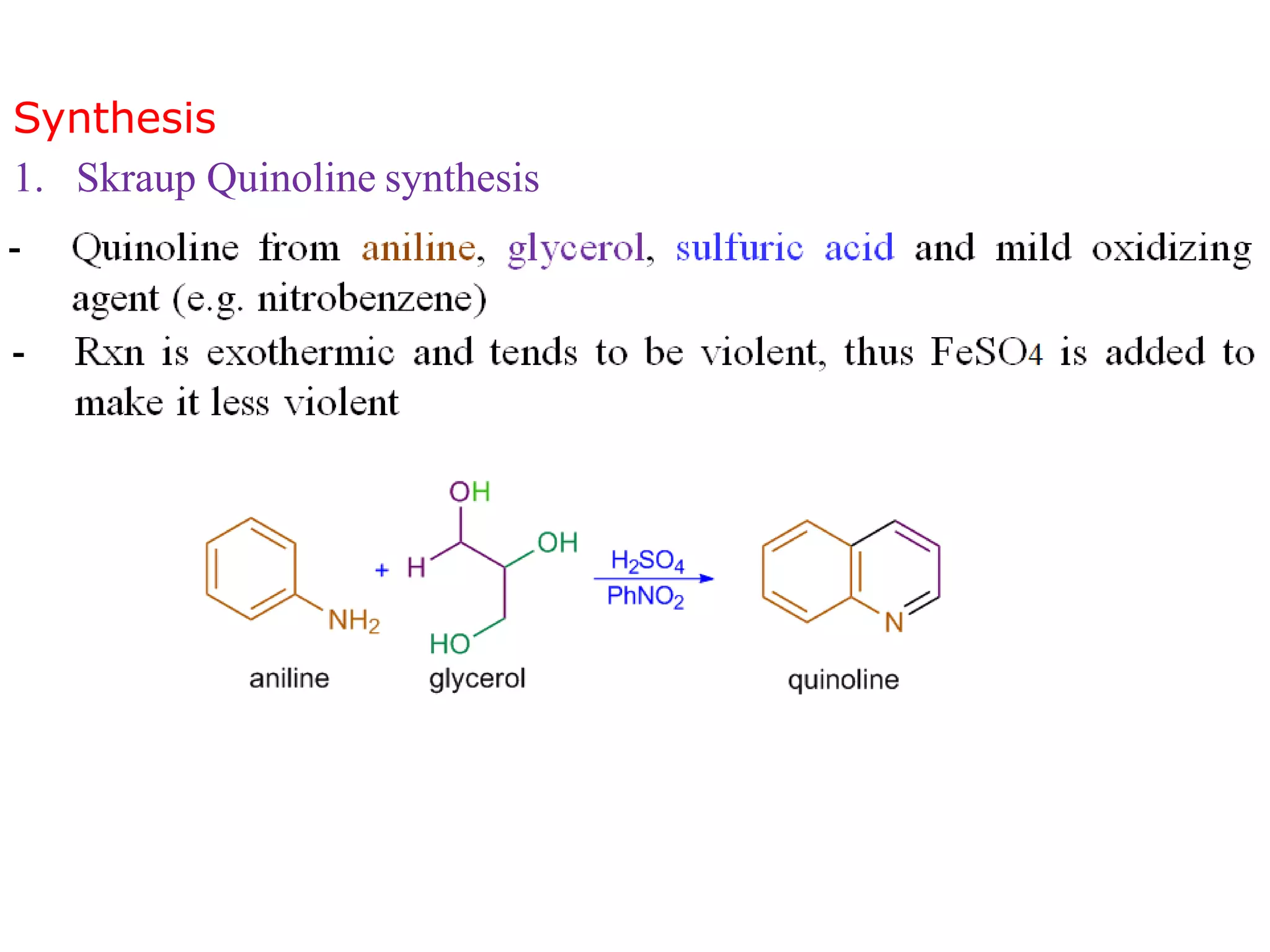
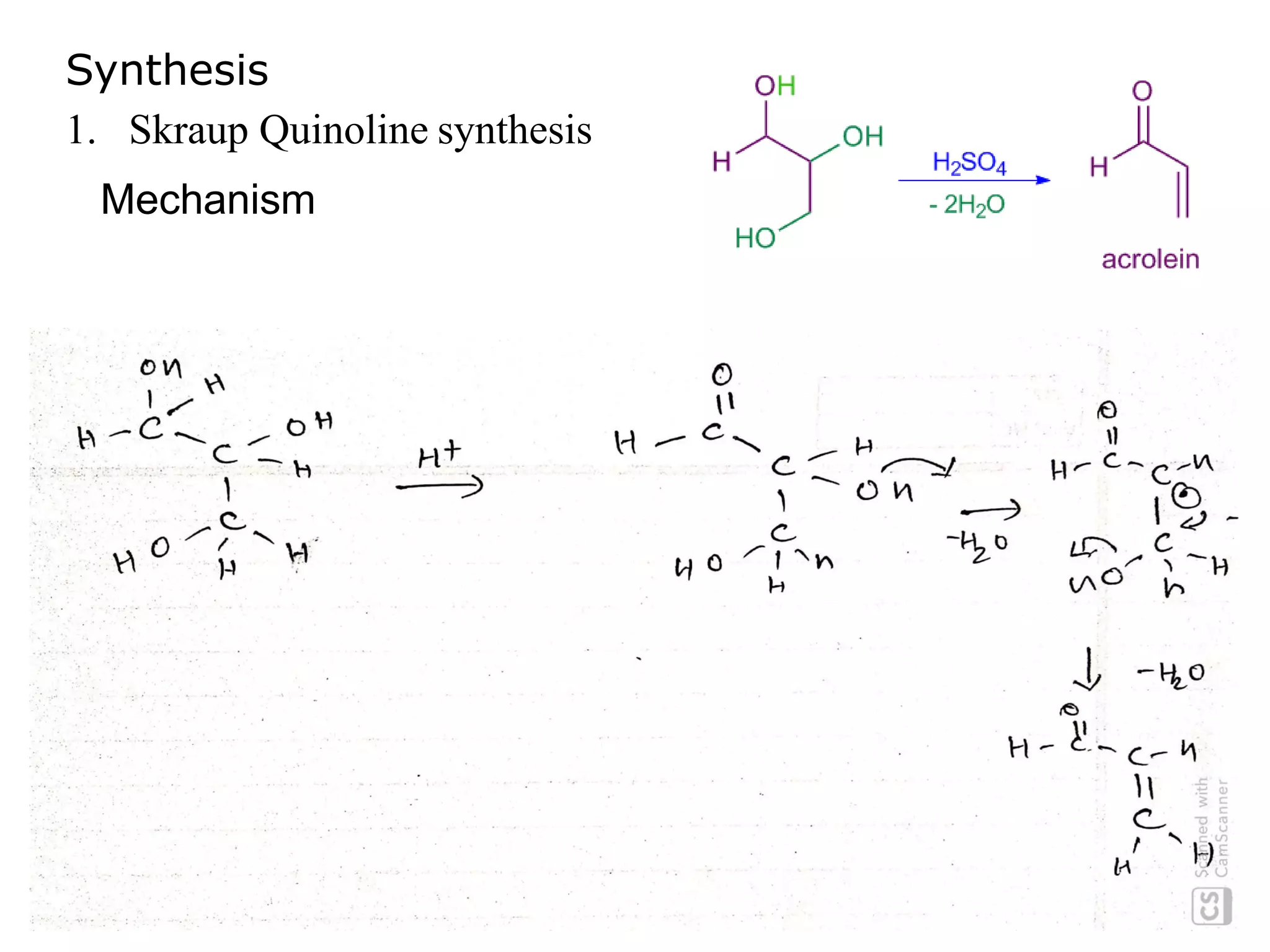
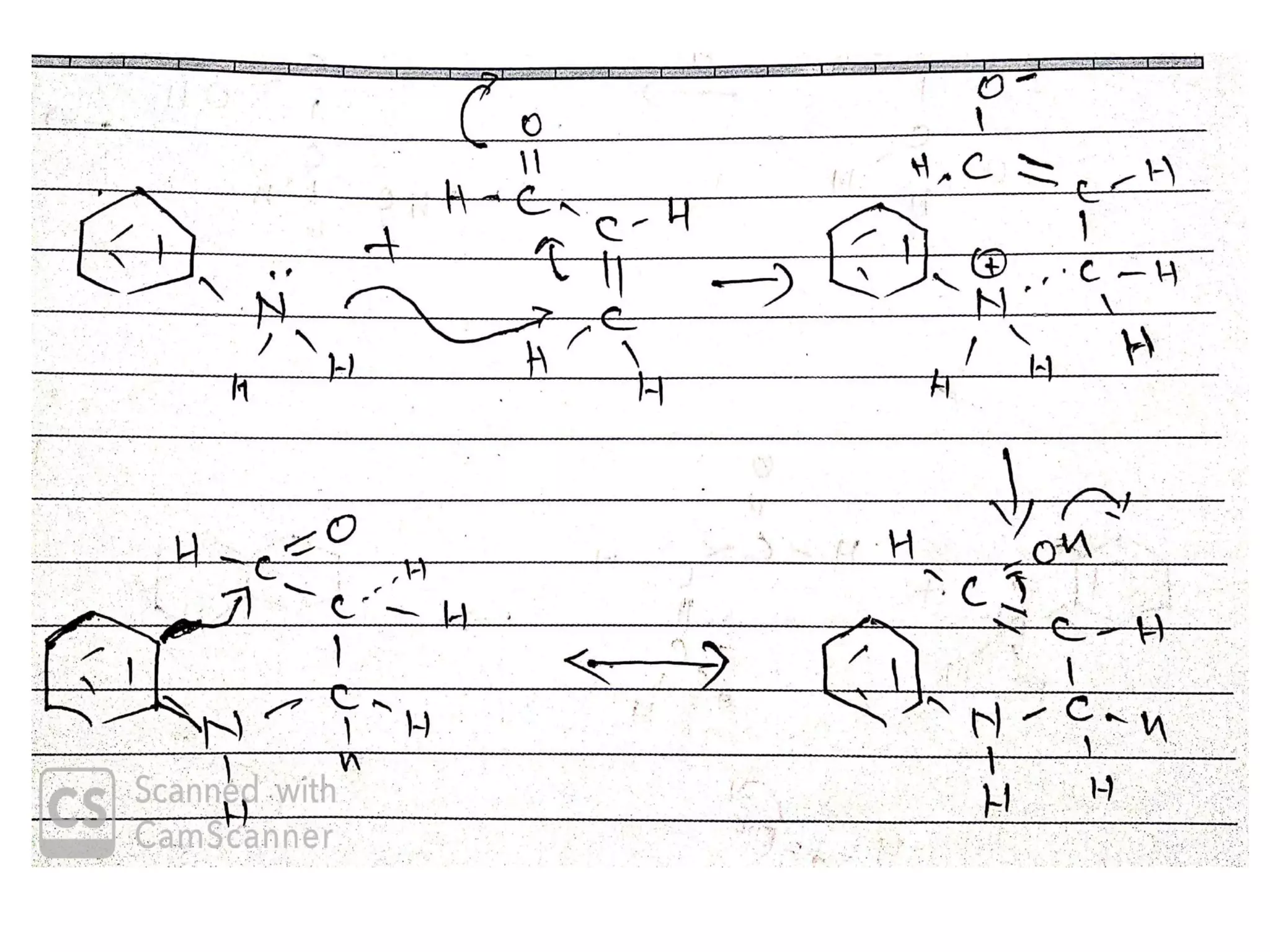
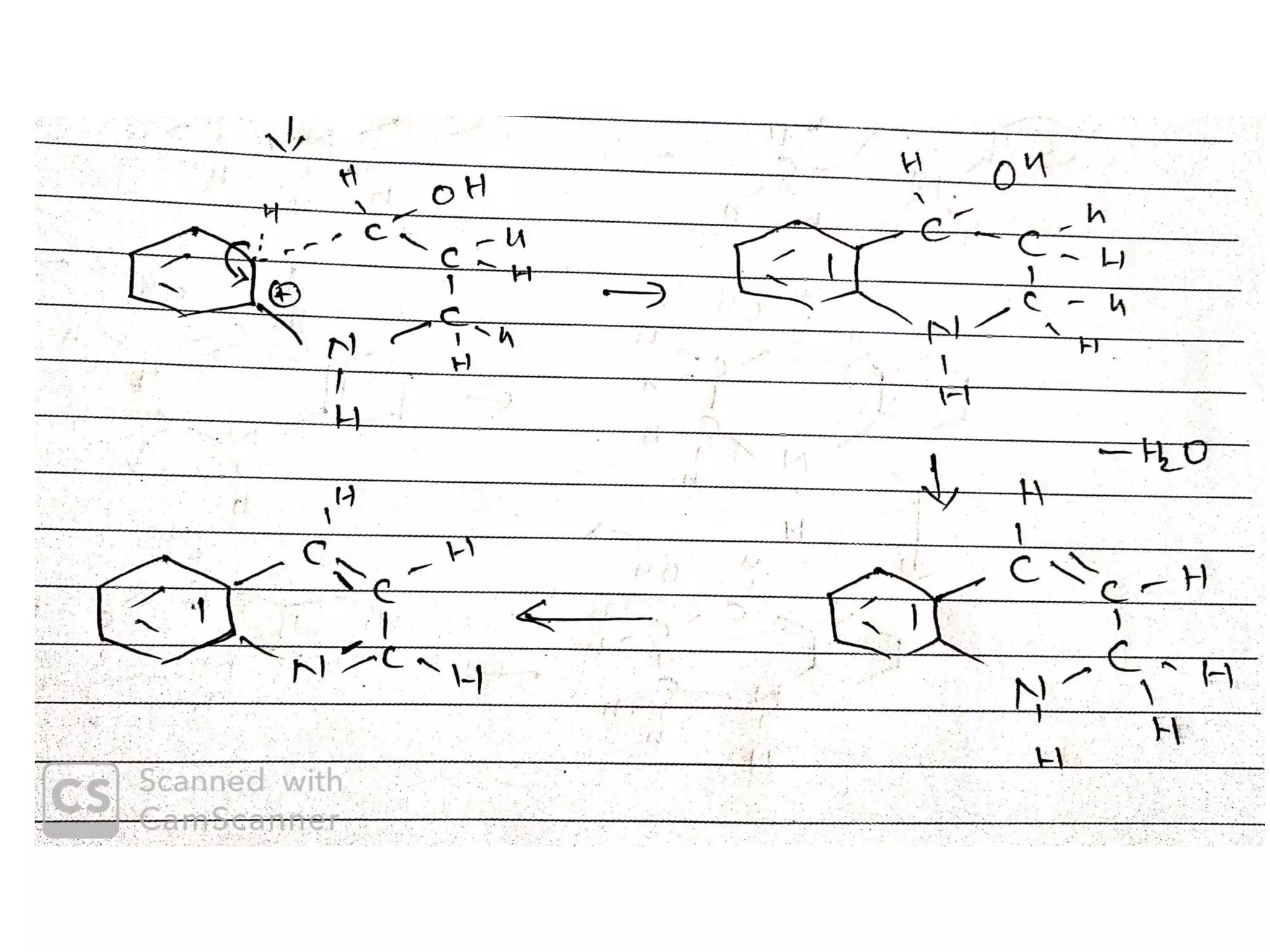
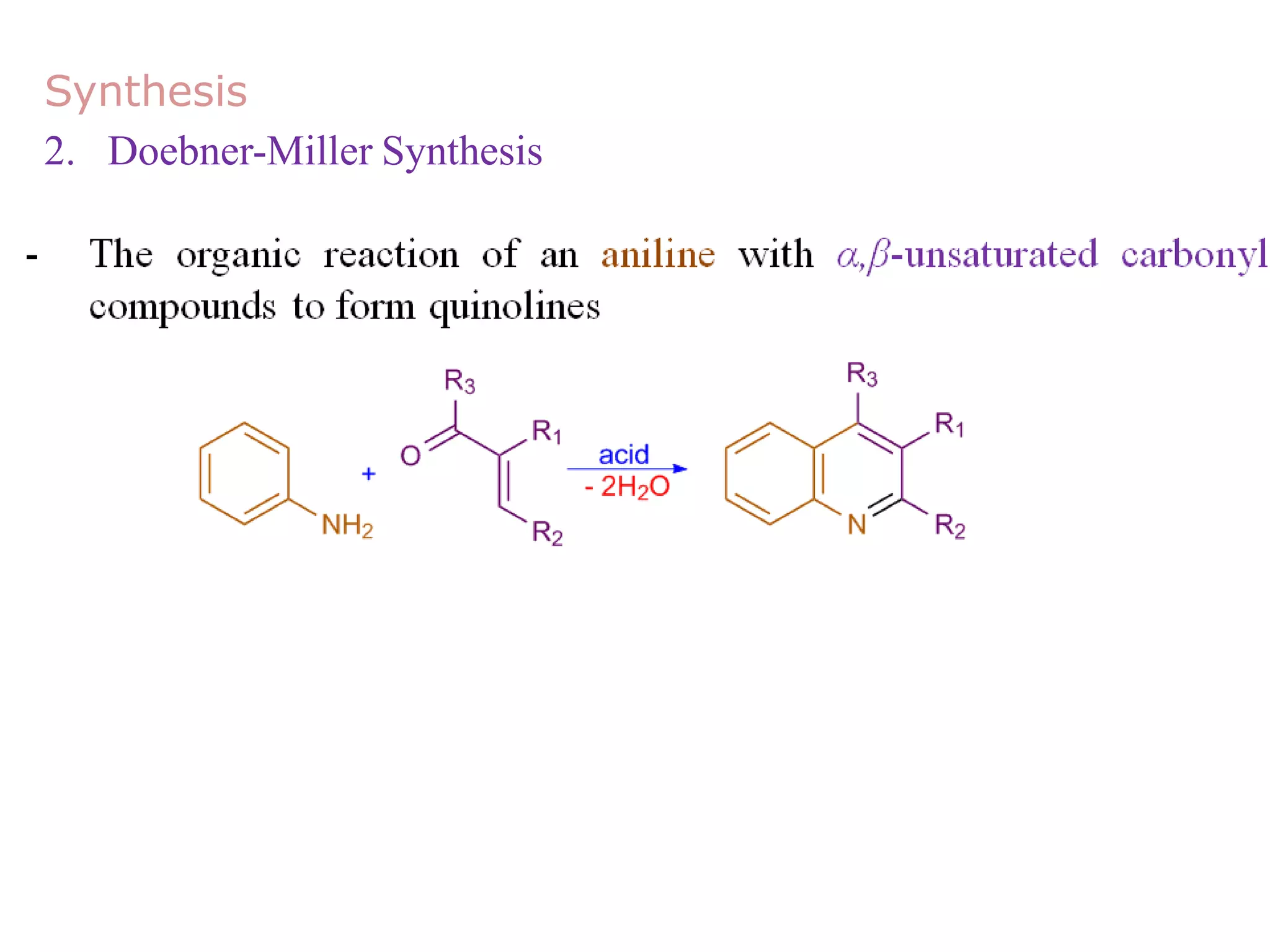
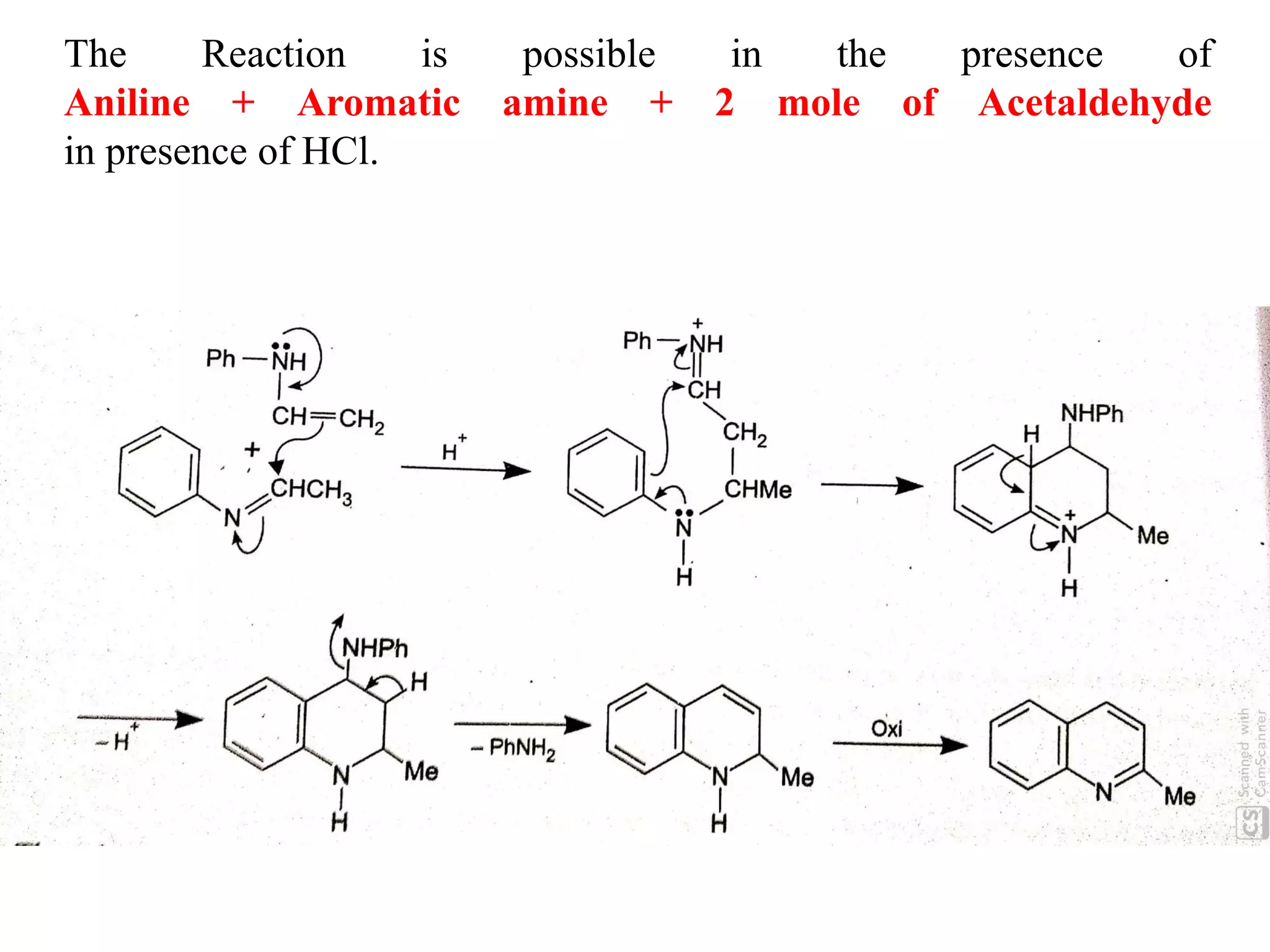
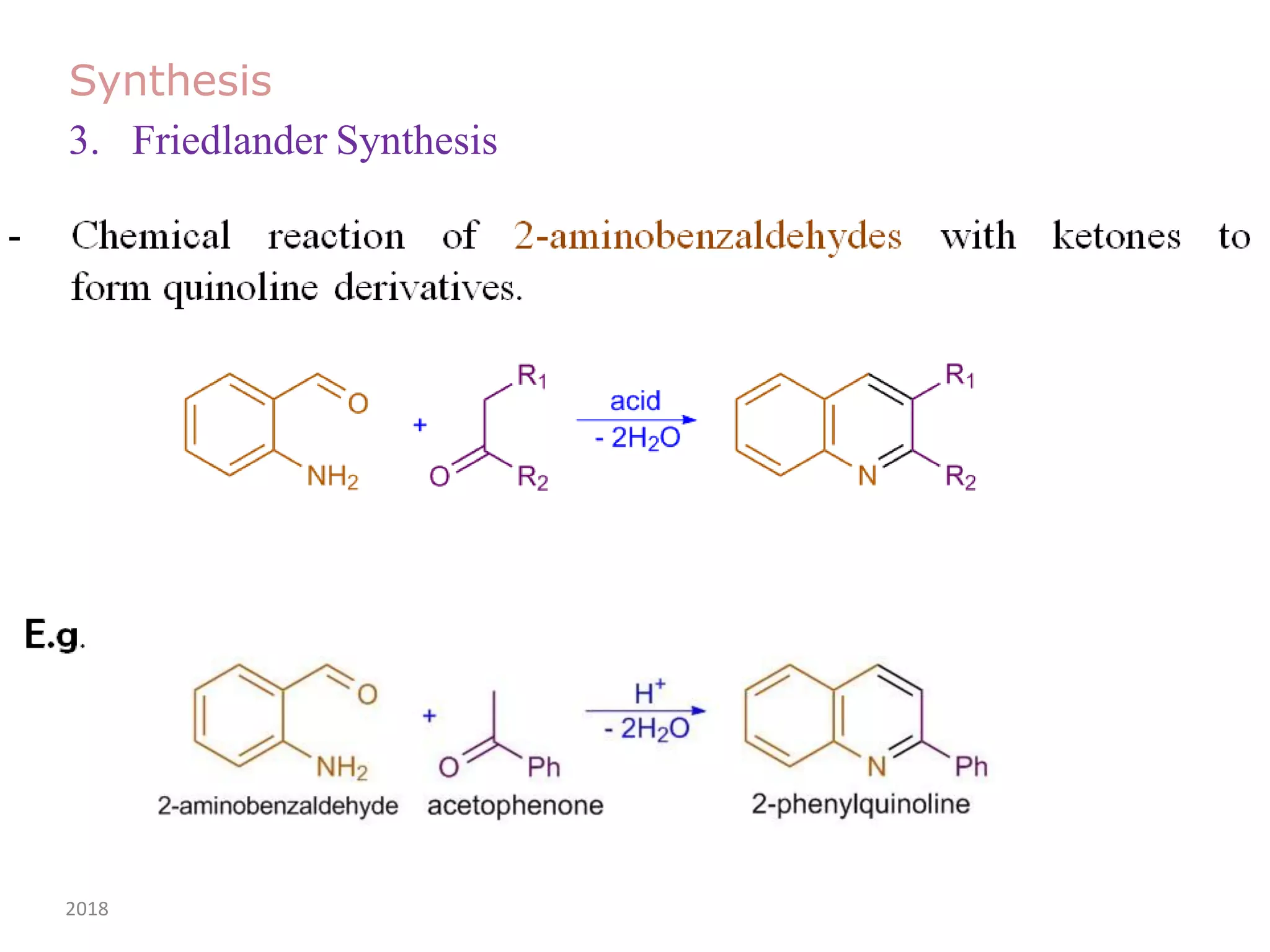



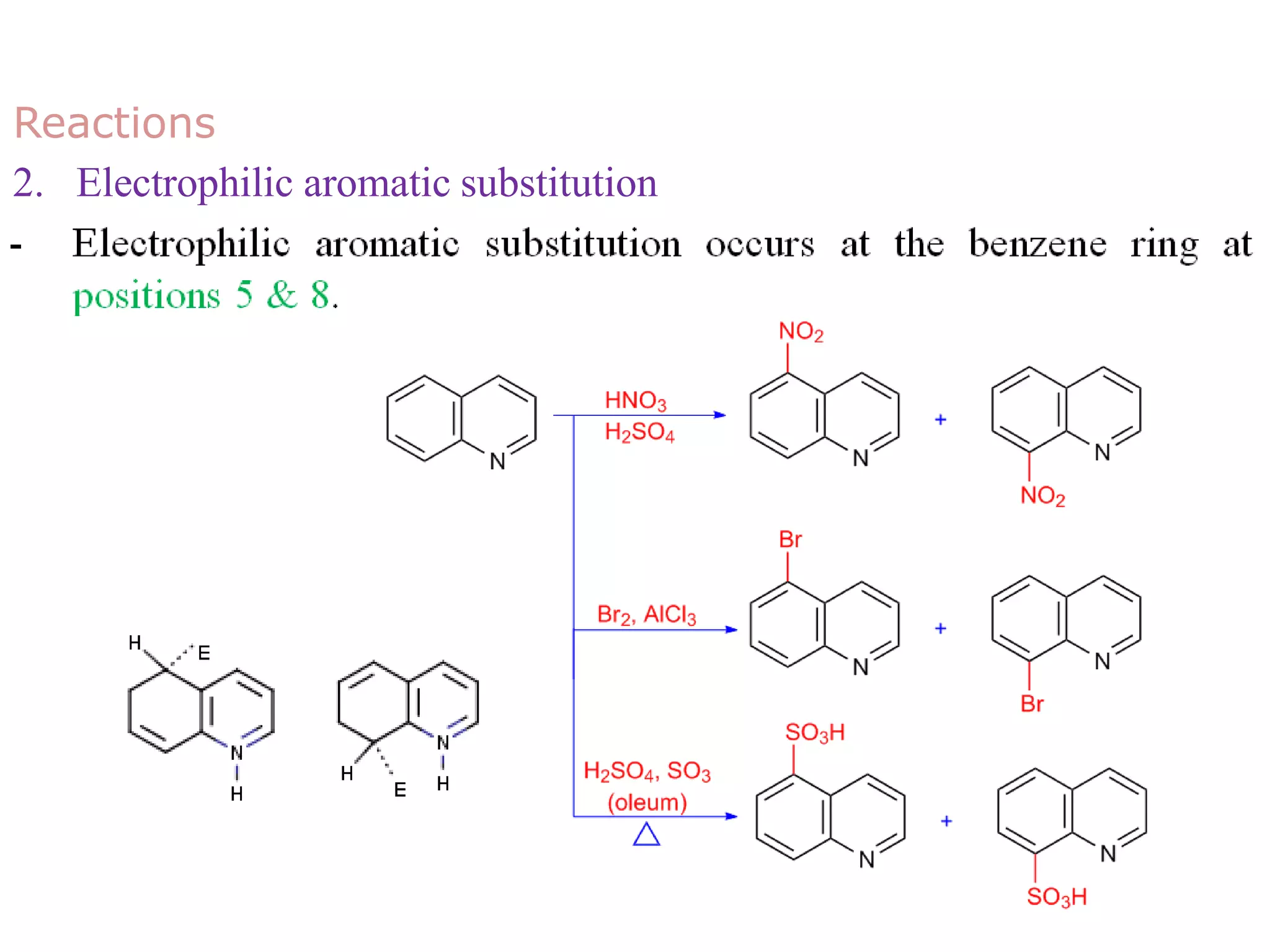

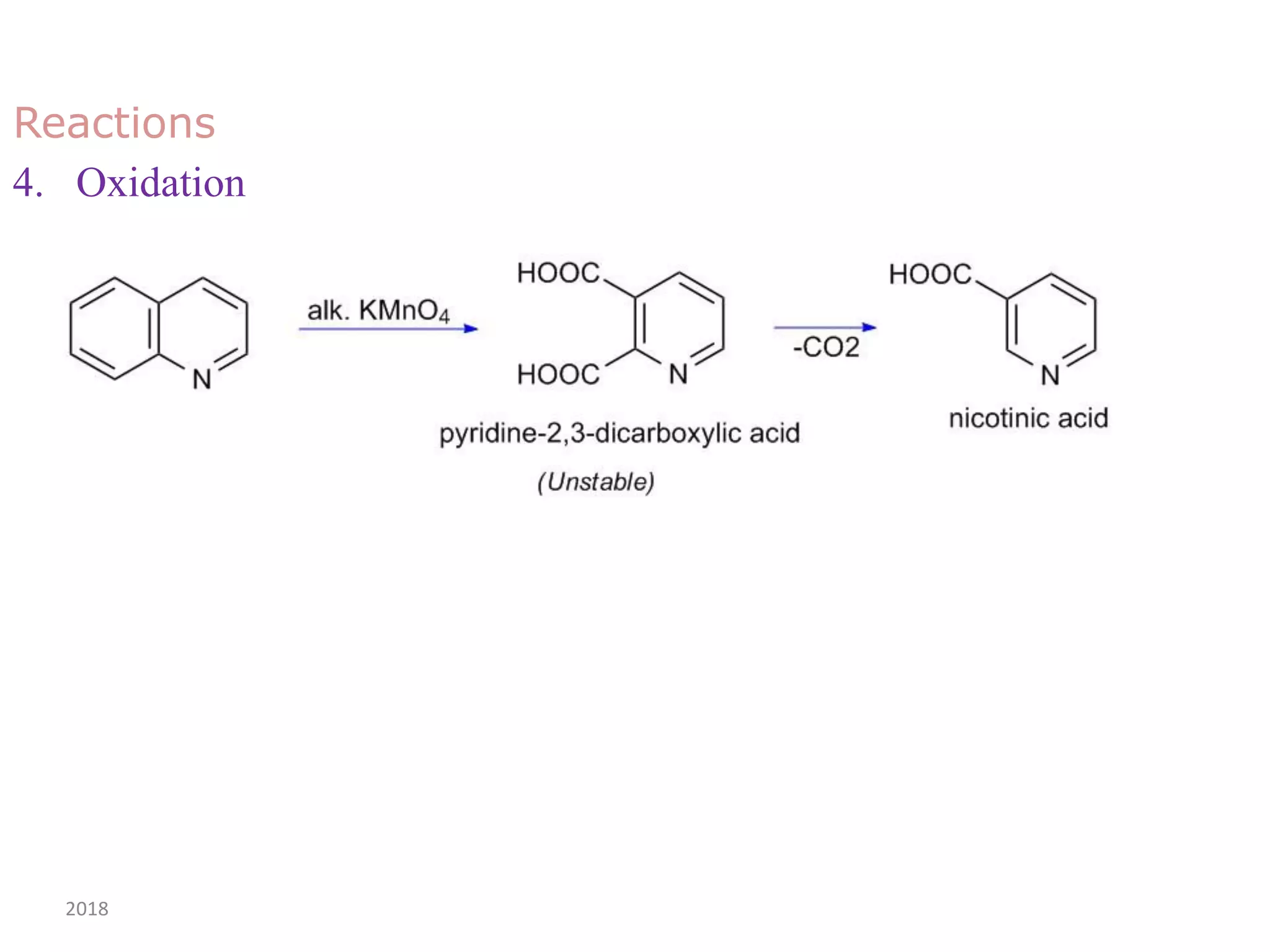
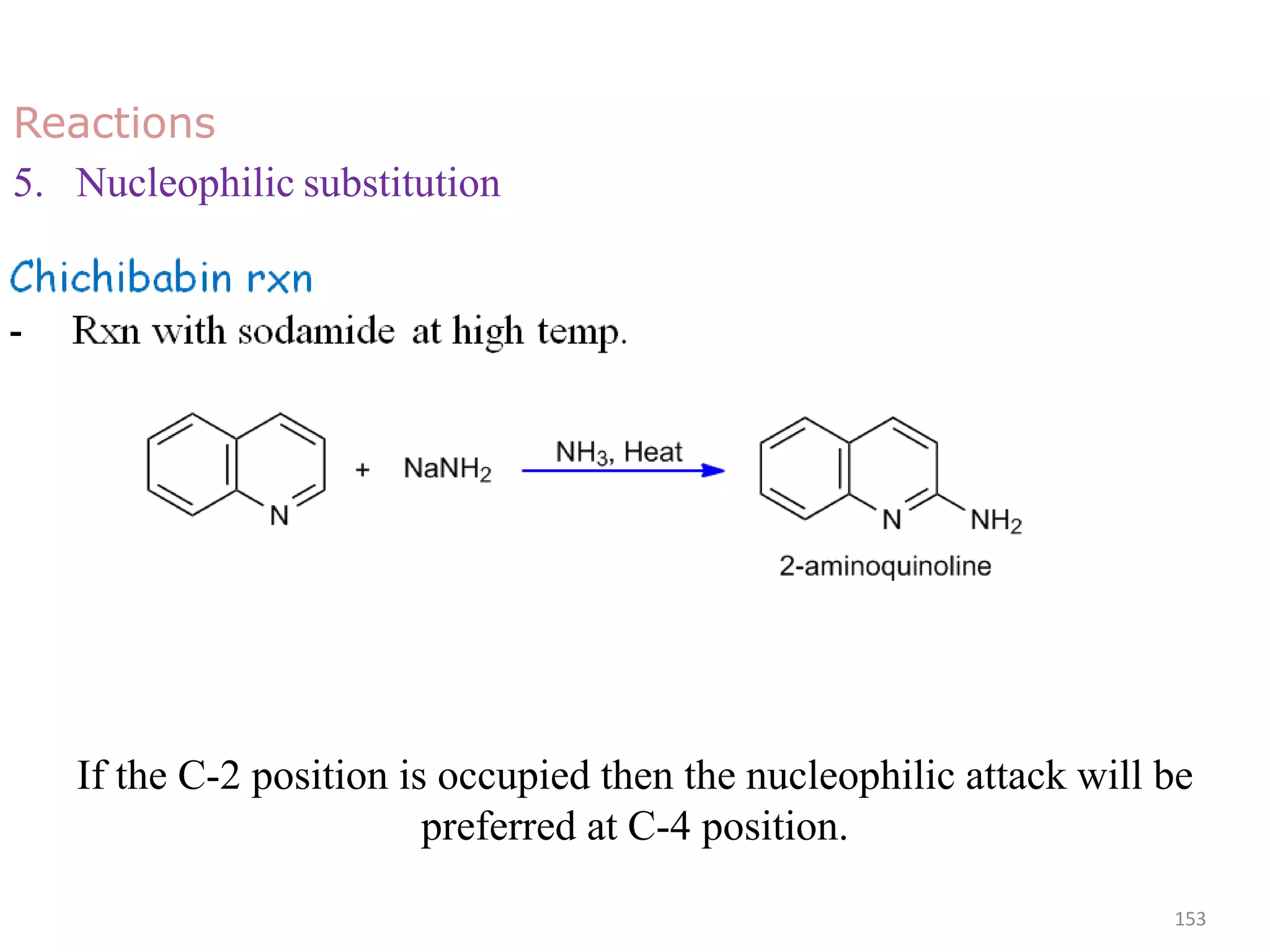
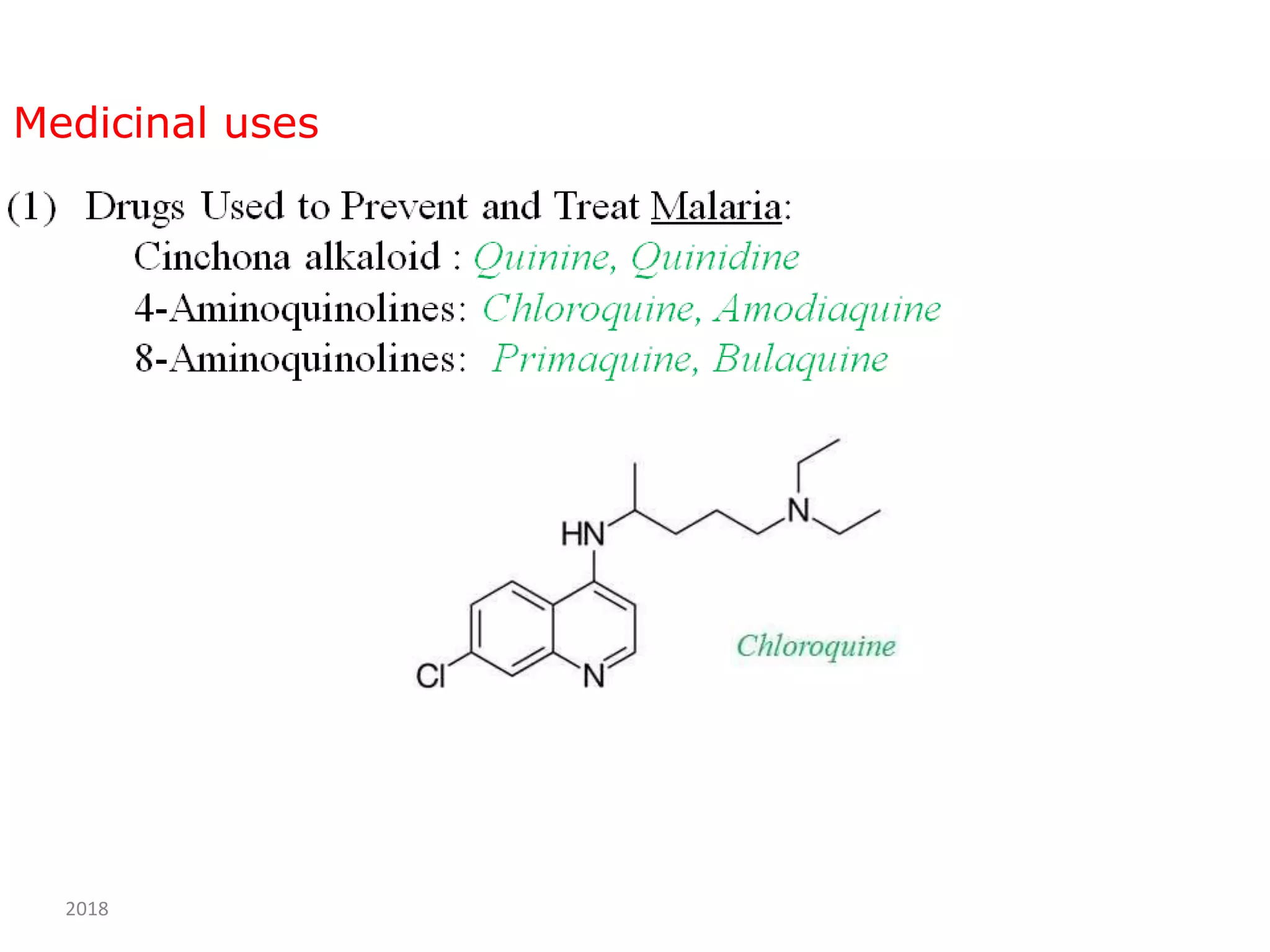
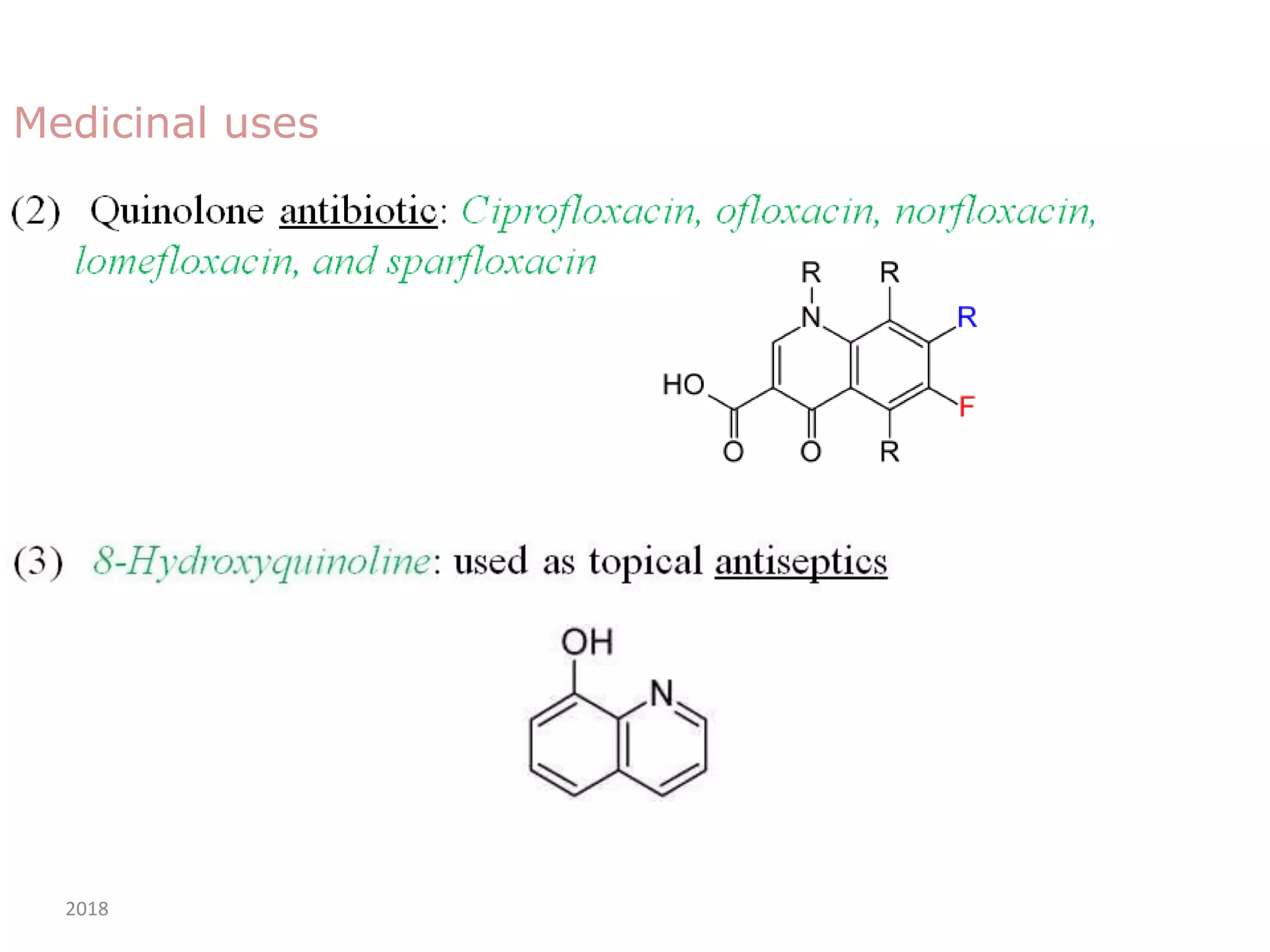
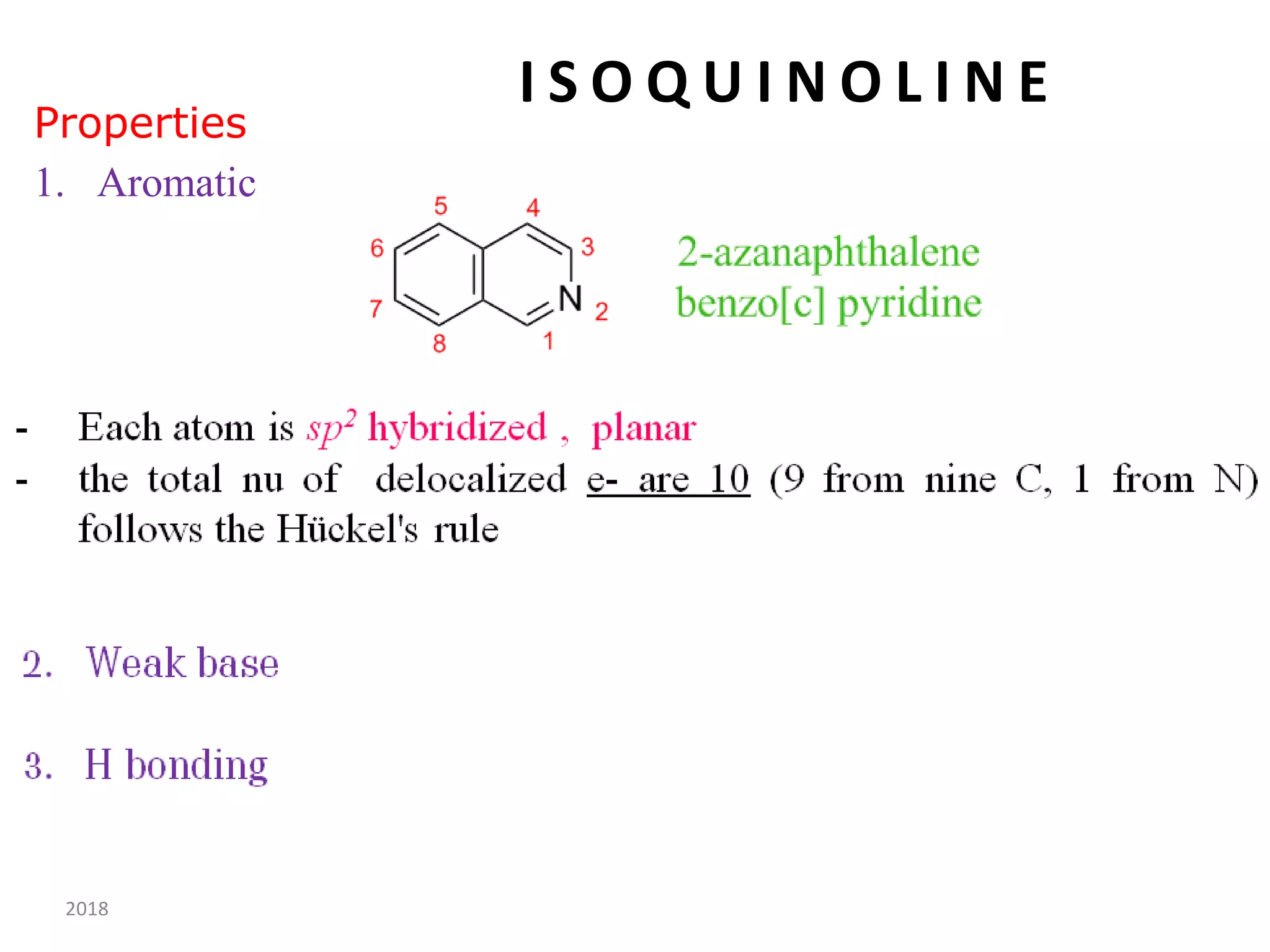
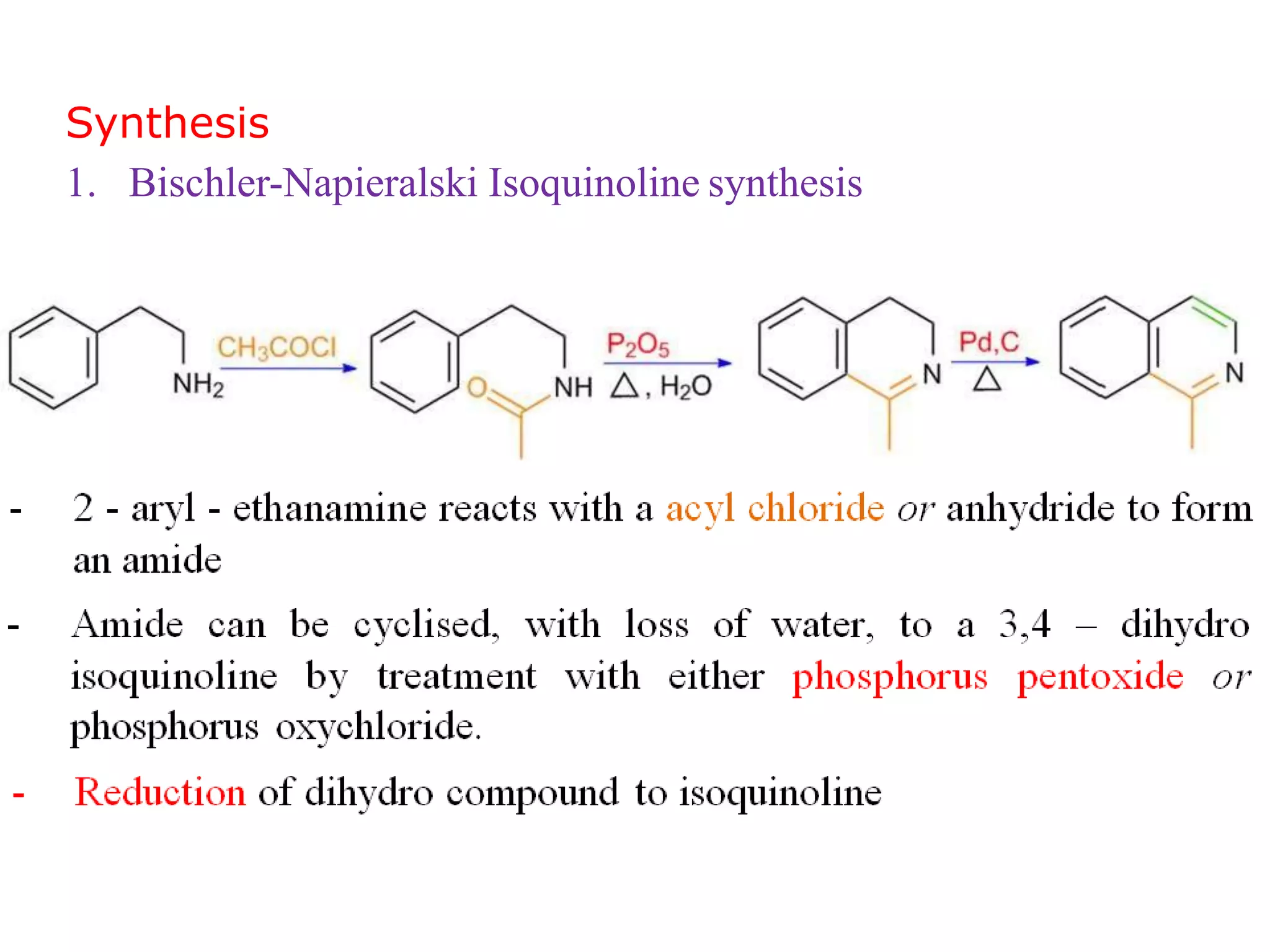

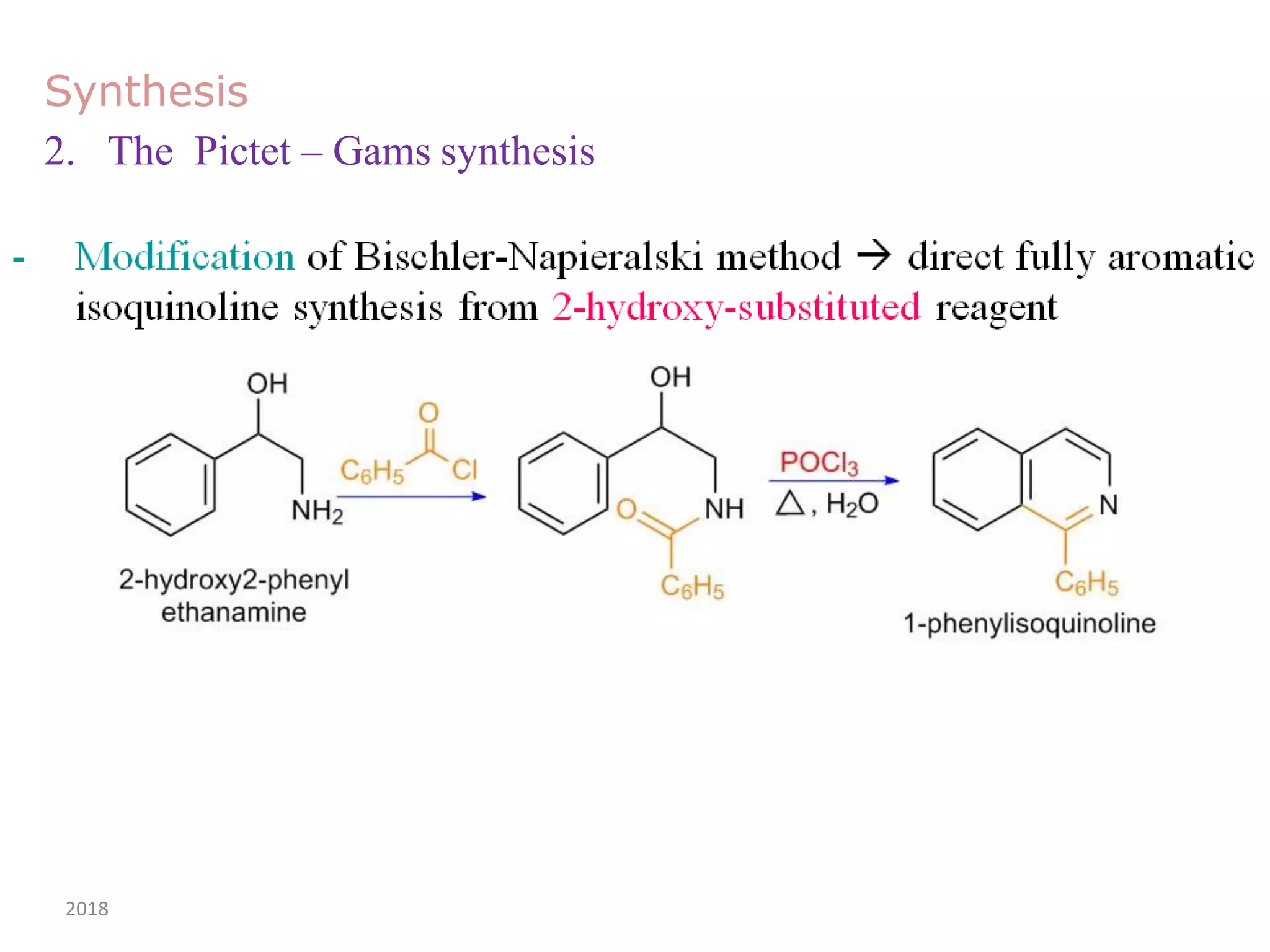
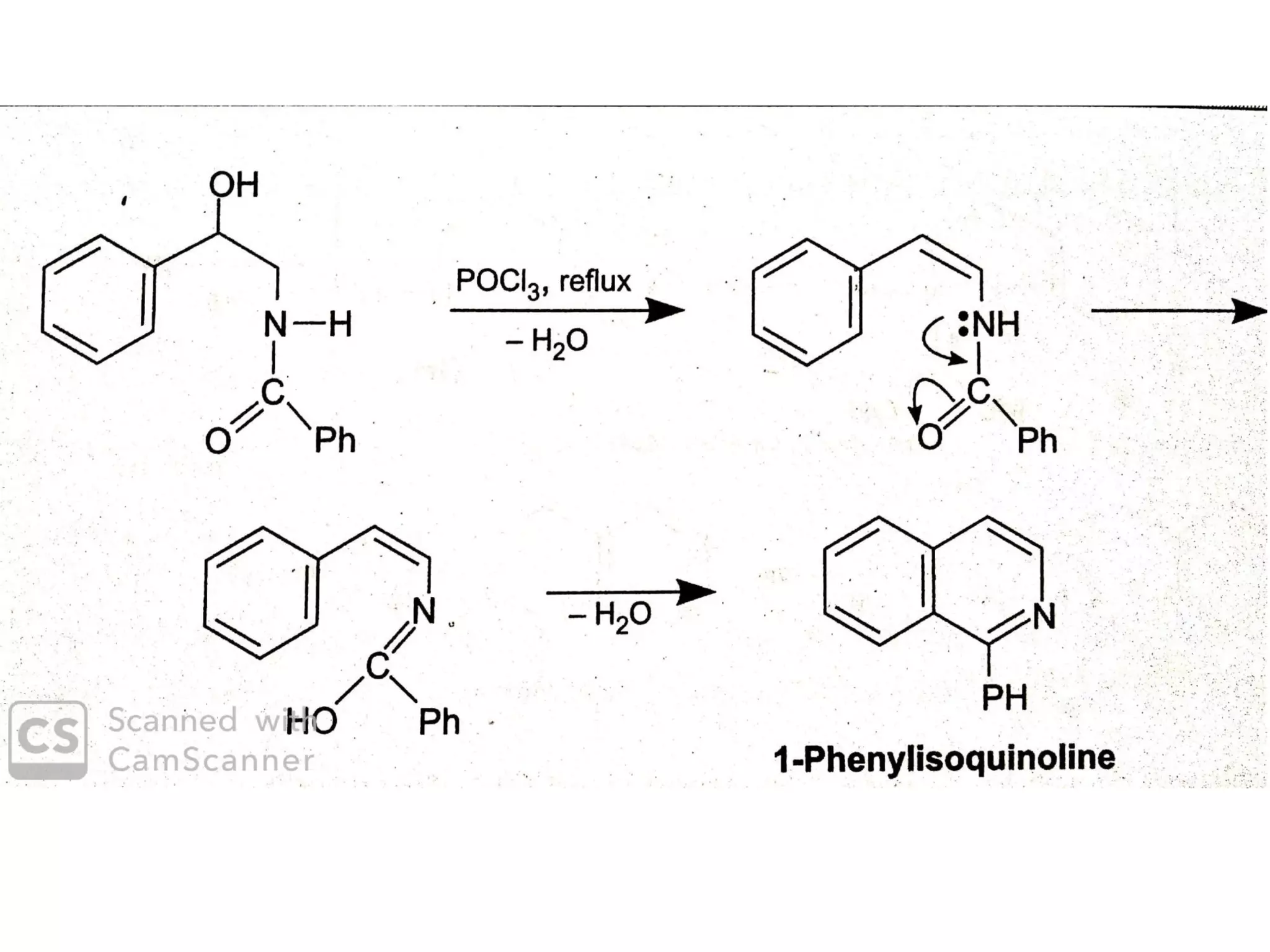
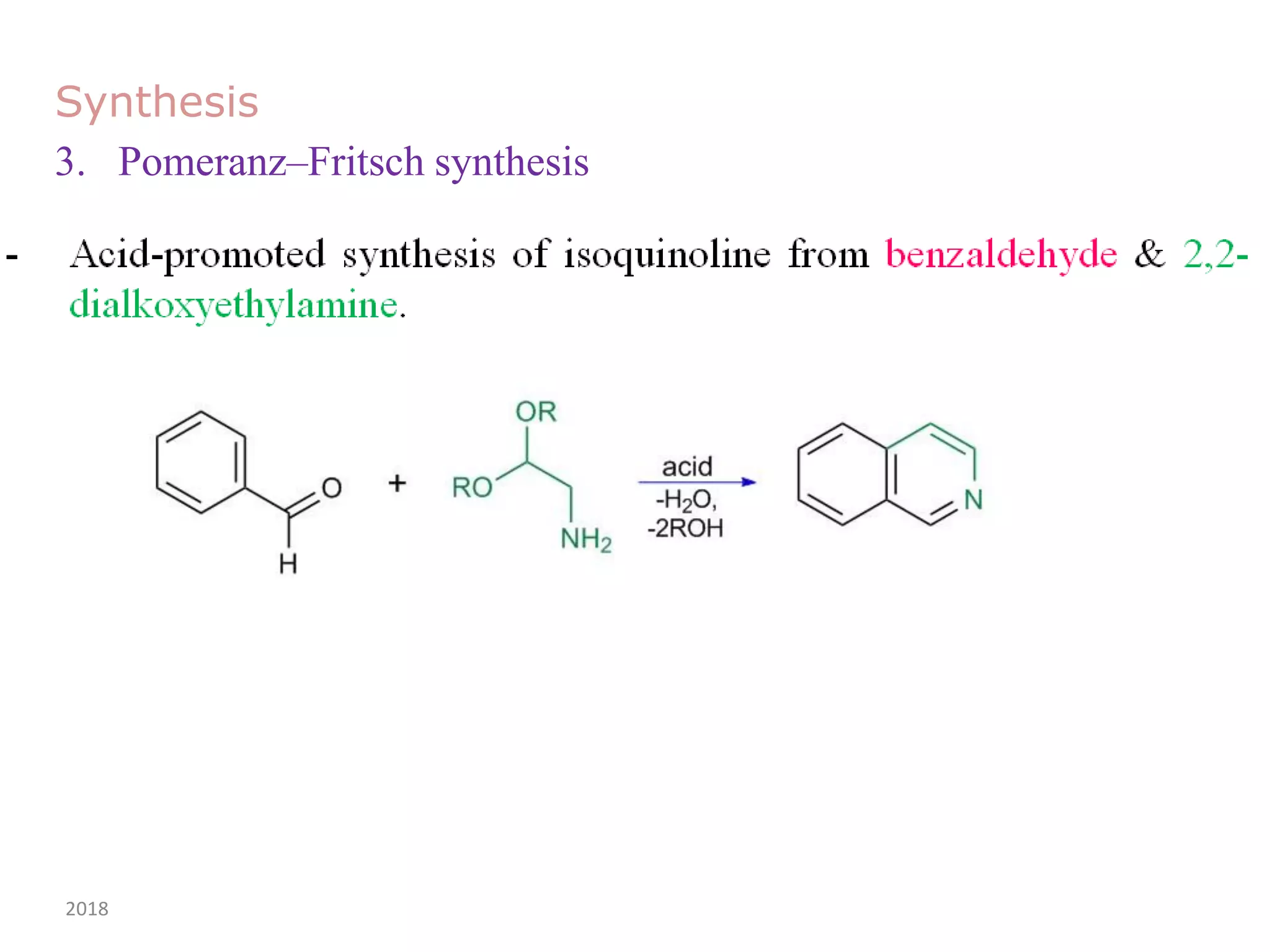



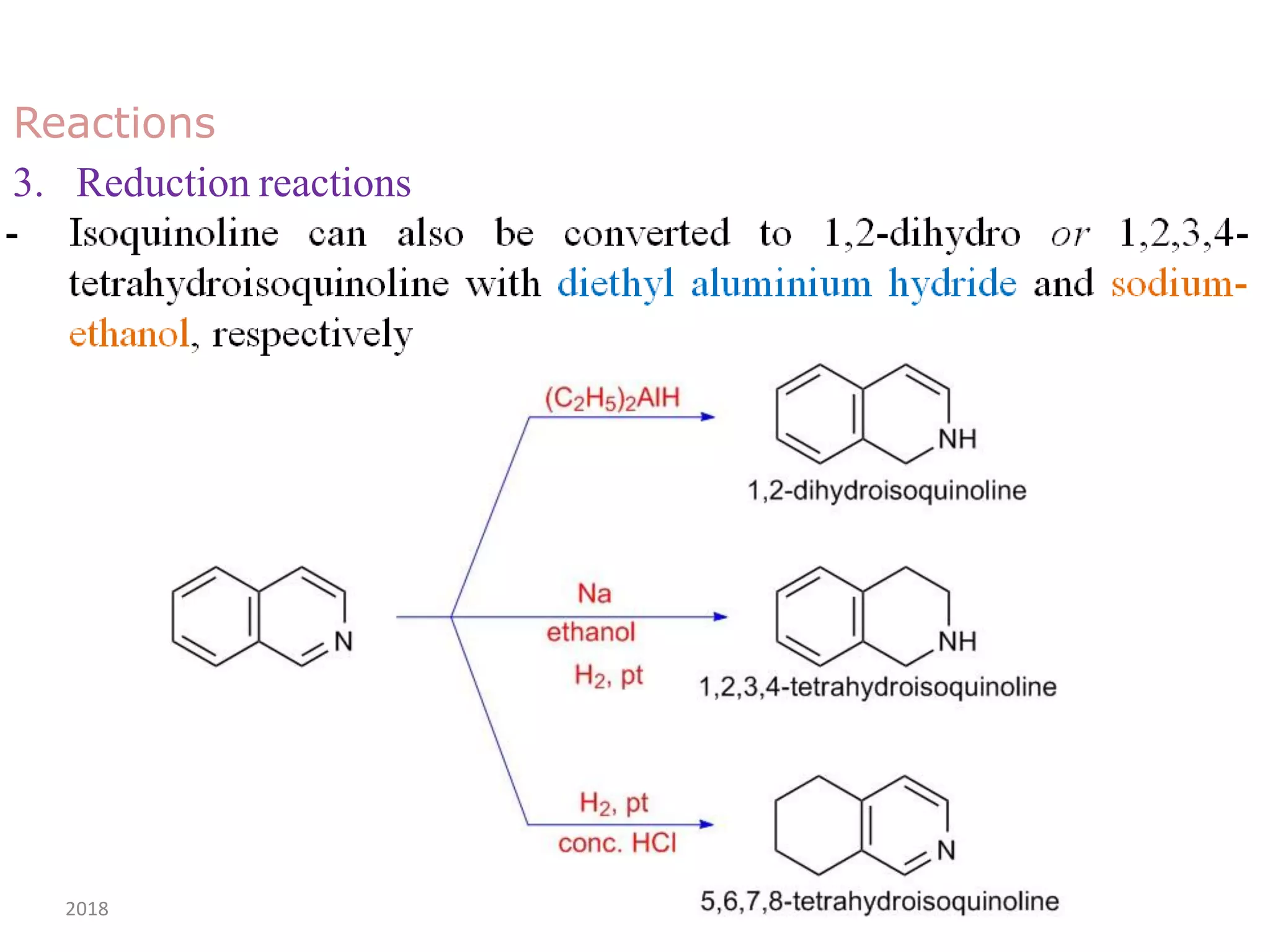
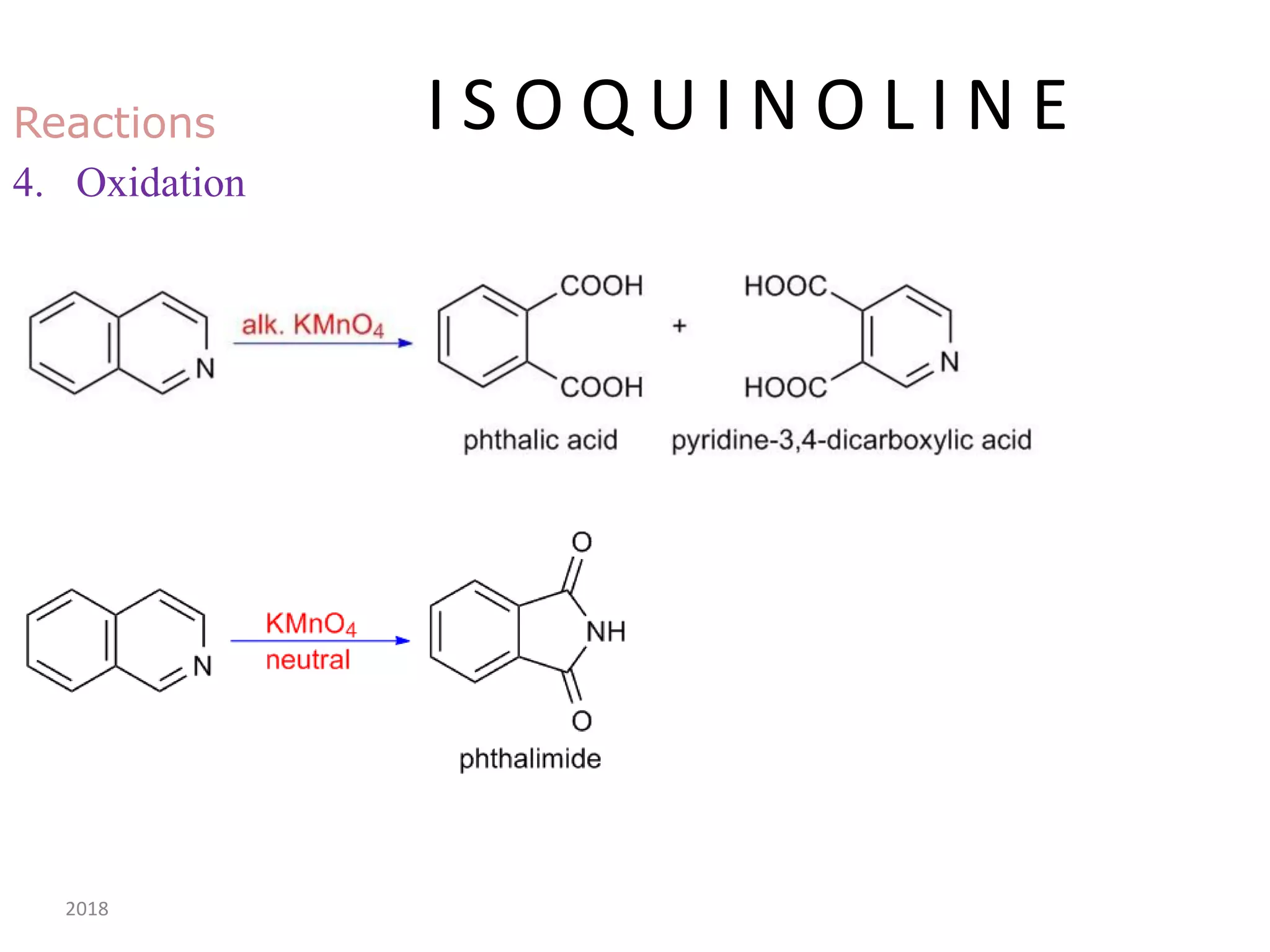
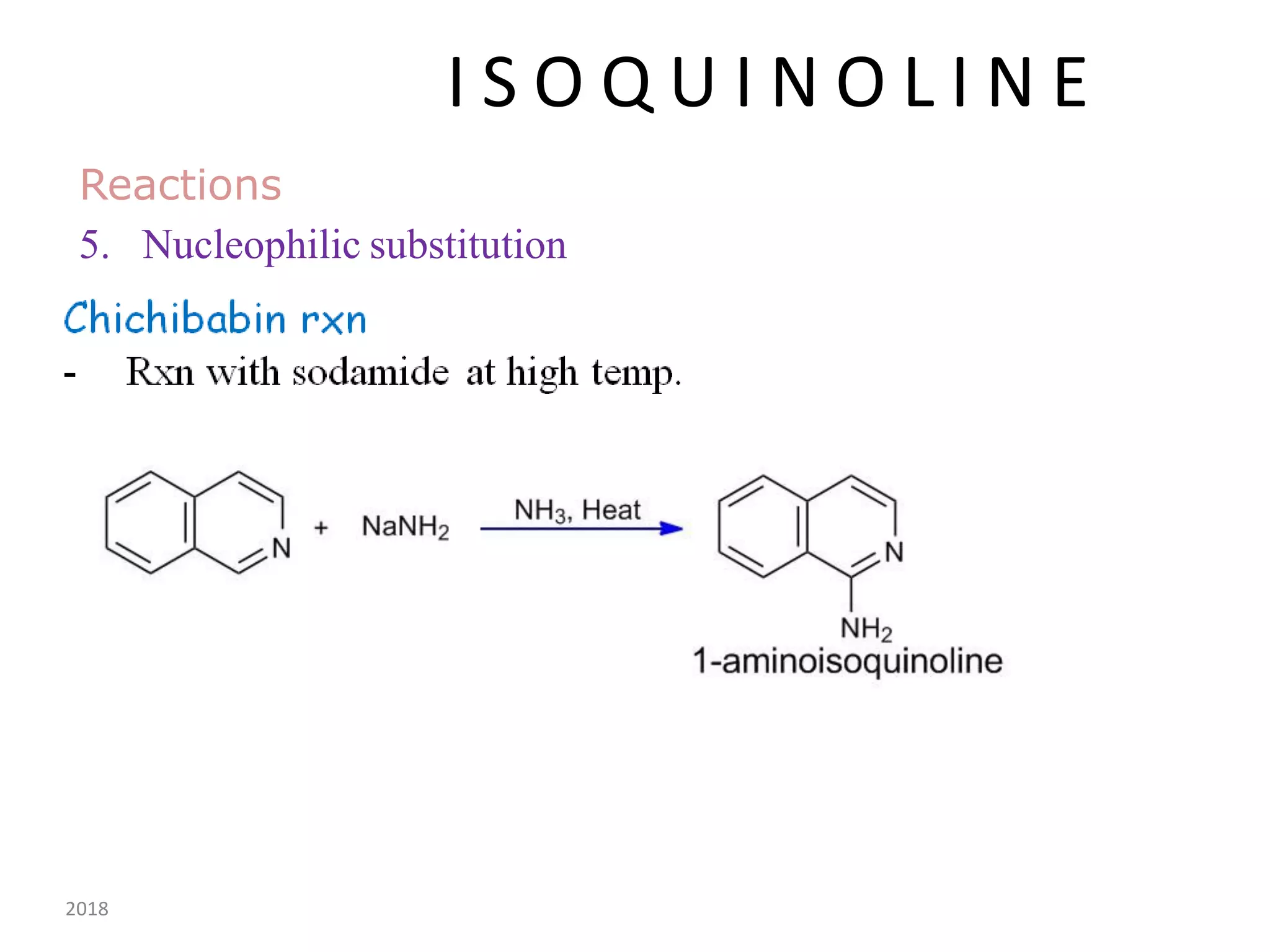

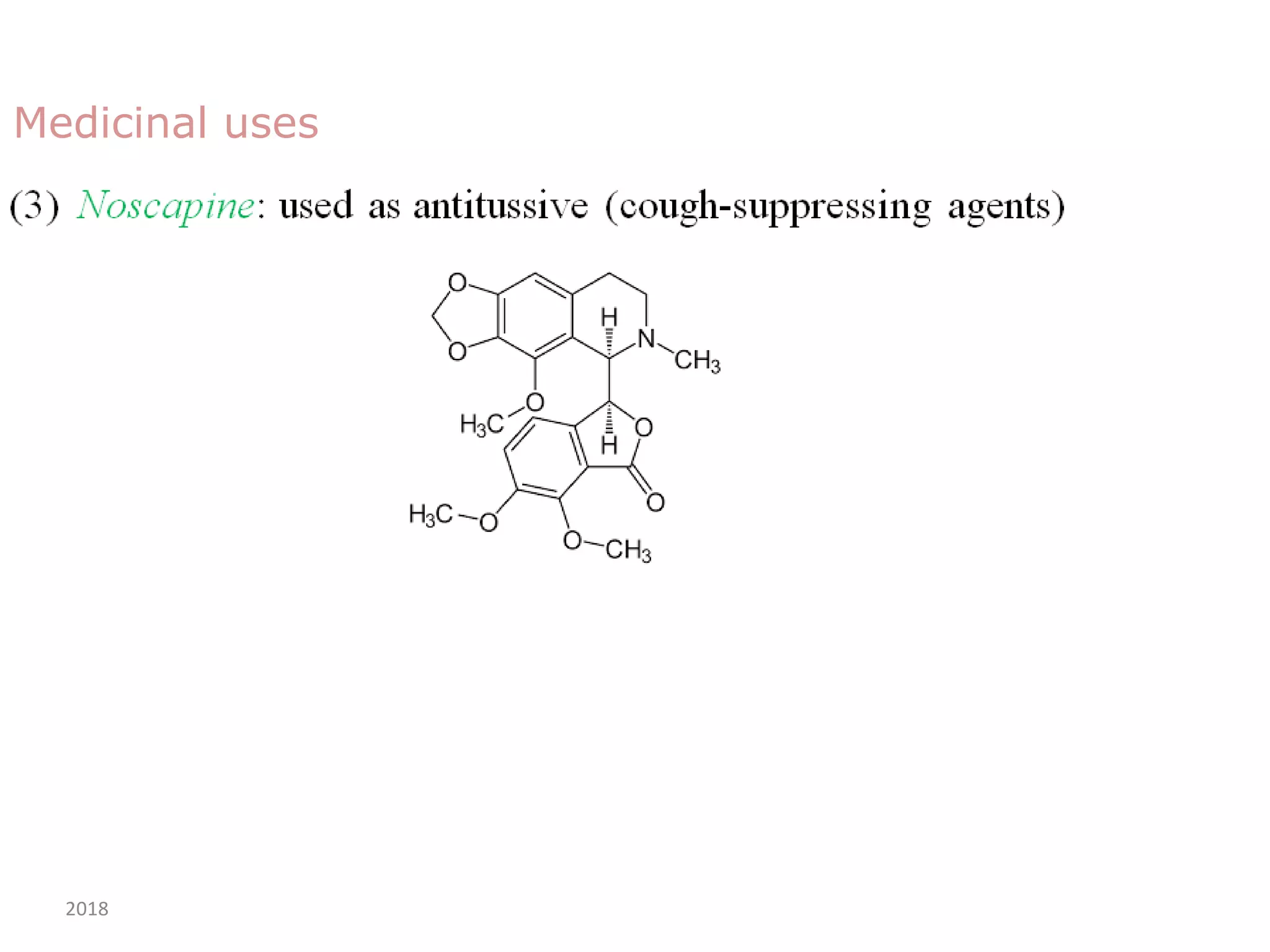

This document discusses the properties, synthesis, and reactions of quinoline and isoquinoline. Quinoline and isoquinoline are aromatic and undergo nucleophilic substitution reactions readily. Key synthesis methods discussed include the Skraup, Doebner-Miller, Friedlander, Bischler-Napieralski, Pictet-Gams, and Pomeranz-Fritsch reactions. The document also outlines various electrophilic addition, substitution, reduction, oxidation, and nucleophilic substitution reactions that quinoline and isoquinoline undergo. Finally, some medicinal uses of quinoline and isoquinoline are mentioned.
































