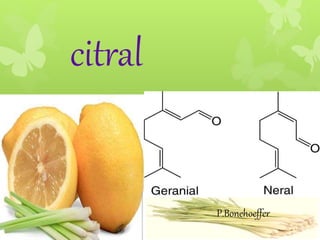
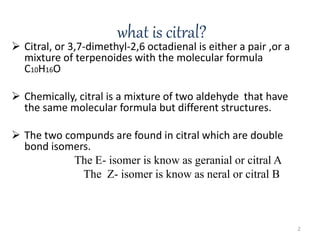
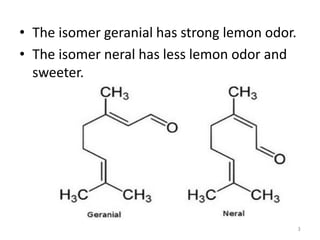
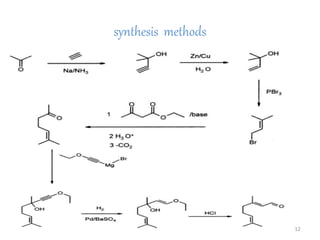
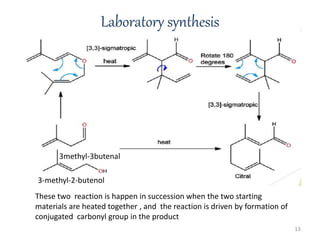
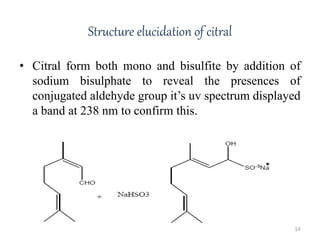
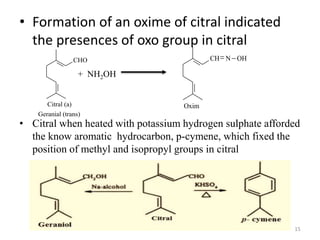
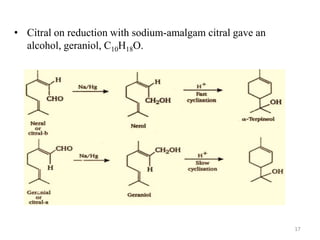
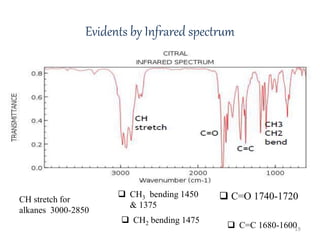
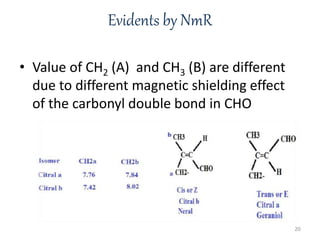
Citral is a mixture of two aldehyde isomers - geranial and neral. Geranial has a strong lemon odor and neral has a less intense lemon odor and is sweeter. Citral is a clear yellow liquid extracted through steam distillation of lemon grass oil. It is used in perfumes, flavors, and insect repellents due to its antimicrobial properties. Laboratory synthesis of citral involves the heating of 3-methyl-3-butenal and 3-methyl-2-butenol. Structure was confirmed through reactions forming derivatives and comparison of products to known compounds.