The document discusses different types of vaccines including:
1. Killed/inactivated vaccines which use killed pathogens.
2. Live attenuated vaccines which use weakened live pathogens.
3. Subunit vaccines which use antigenic subunits of pathogens.
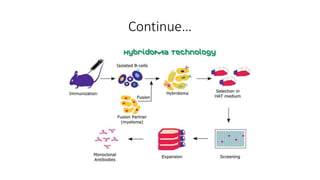
4. Peptide vaccines which are synthesized peptides or recombinantly produced.
5. Toxoid vaccines which use inactivated bacterial toxins.
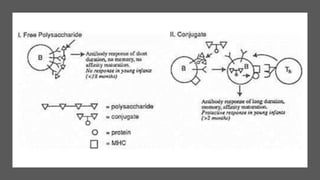
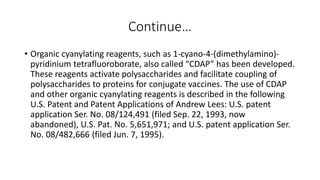
6. Conjugate vaccines which combine weak polysaccharide antigens with strong protein carriers.
7. Recombinant vector vaccines which use attenuated viruses to deliver genetic material.
8. Anti-idiotypic vaccines which mimic pathogen epitopes to induce an immune response.
![Killed vaccine/inactivated
vaccine
• An inactivated vaccine (or killed vaccine) is a vaccine consisting
of virus, bacteria, or other pathogens that have been grown
in culture and then killed using methods.
• Physical method: pasteurization methods / heating.
• Chemical method: Formalin , beta-Propiolactone (BPL) , binary
ethylenimine (BEI) .
. [Avian Dis. 1991 Jul-Sep;35(3):505-14.]
• Typhoid vaccine is a bacterium based inactivated vaccine.
[Vaccine Types, 2017.]](https://image.slidesharecdn.com/vaccinepreparation-190104174210/85/Vaccine-preparation-part-2-2-320.jpg)




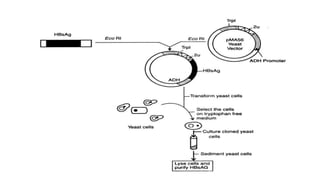

![Peptide vaccine
• A peptide vaccine is any peptide which serves to immunize an
organism against a pathogen.
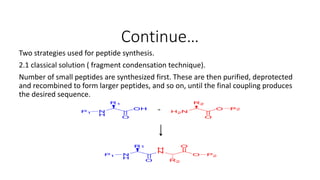
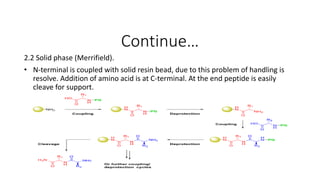
• Two ways of making peptide vaccine.
1. Recombinant technique in which DNA sequence is express in
suitable vehicle. Mostly use for peptide which has more then 50
residue.[WHO, technical report series, No:889, 1999]
2. Synthesis of peptide: sequence reaction. Use for peptides having
residues less then 50.](https://image.slidesharecdn.com/vaccinepreparation-190104174210/85/Vaccine-preparation-part-2-9-320.jpg)

![Toxoid vaccine
• Toxoid is a chemically inactivated toxin. Toxoid vaccines are little
different from the vaccines discussed previously. They use the germs
toxin that causes a disease.
• Diphtheria toxoid is prepared by formaldehyde treatment of toxin
and is standardized for potency according to the U.S. Food and Drug
Administration.
• Toxoid is adsorbed to aluminum salts, which enhances
immunogenicity.
[Irini Daskalaki, in Principles and Practice of Pediatric Infectious Diseases
(Fourth Edition), 2012]](https://image.slidesharecdn.com/vaccinepreparation-190104174210/85/Vaccine-preparation-part-2-13-320.jpg)