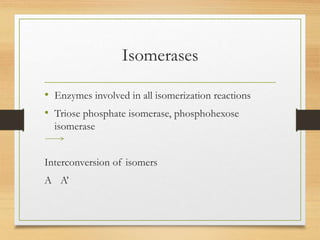
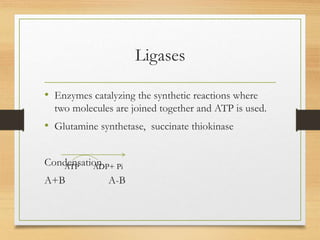
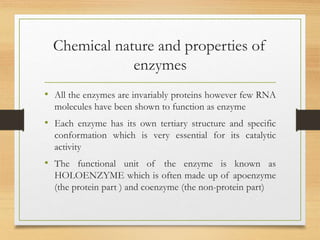
Enzymes are biocatalysts synthesized by living cells that accelerate chemical reactions without altering themselves. They are classified into six major categories based on the type of reactions they catalyze, encompassing a wide range of functions specific to various substrates. Enzymes are predominantly proteins, with specific structures essential for their activity, often consisting of an apoenzyme and a coenzyme.