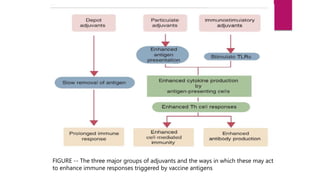
This document discusses adjuvants, which are compounds added to vaccines to stimulate stronger immune responses. It defines adjuvants and classifies them into delivery systems (particulate adjuvants) and immune potentiators. Major particulate adjuvants discussed are alum, Freund's incomplete adjuvant, MF59, and AS03. Major immune potentiators discussed are TLR agonists. The document also covers mucosal adjuvants, licensing of adjuvanted vaccines, and concludes that rational adjuvant selection is important for vaccine design.




![Classification of adjuvants :
Adjuvants can be classified according to their
physicochemical properties, origin, and mechanism of action.
Based on their mechanisms of action, adjuvants can be
divided into delivery systems[particulate] and immune
potentiators[immunostimulatory].
Mucosal adjuvants are a class of compounds that can fit in
both of the previously described categories.](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-5-320.jpg)

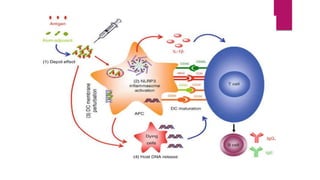
![DEPOT ADJUVANTS
Alum :
the most used adjuvant since its introduction is in 1920s.
This ajuvant is in the formulation of licensed vaccines against Hepatitis A,B,
diptheria/tetanus/pertussis, human papilloma virus, HiB AND PNEUMOCOCCUS.
They are sensed by NOD like receptors through direct activation of NLRP3
inflammasome complex by release of uric acid.
These preferentially induce Th2 responses, and for some pathogens a Th1
immune response[including cytotoxic CD8 Tcells]. Because of this it doesn’t elicit
protective and sustained IR for some vaccines.](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-8-320.jpg)


![PARTICULATE ADJUVANTS
Ex: Emulsions,microparticles, immune stimulating complexes[ISCOMs],
liposomes.
These deliver antigens to APCs
Easily entocytosed due to particle size similar to bacteria.
ISCOMs – these are complex lipid based micrparticles, stimulate either
Th1 or Th2 response.
These are not widely used in veterinary vaccines.](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-12-320.jpg)

![ AS03:
Oil-in-water emulsion that contains alpha
tocopherol, squalene and polysorbate.
It stimulates the immune system by the activation
of NF-Kb, proinflammatory cytokine and
chemokine production, recruitment of immune
cells[mainly monocytes and macrophages], and
induction of high antibody titres.](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-14-320.jpg)
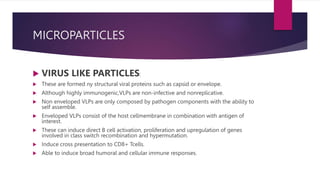
![ VIROSOMES:
Composed of reconstituted viral envelopes with membrane lipids and viral
glycoproteins
They are not virulent because the genetic material of native virus is absent.
These can increase the expression of costimulatory molecules[CD80, CD86 AND
CD40] on the APC surface.
Leads to CD8+, CD4+ T cell activation and cytokine production such as IFN
gamma, TNF alpha and GM-CSF.
PLA/PLGA:
Biodegradable and biocompatible.
Efficiently reach MHCI molecules and cross present antigens to CD8+ Tcells.
Prolonged release of antigen and higher IR.
PLGA has been used as delivery system for Bacillus anthracis, HBV, Plasmodium vivax.](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-16-320.jpg)

![IMMUNE POTENTIATORS
THESE target innate immunity signalling pathways through PRRs like TLRs,
RLR,NLRs.
Activation of PRRs by their agonists induce APC activation and cytokine/
chemokine production.leads to adaptive immune responses.
Examples :
TLR3 agonists- POLY[I:C]
TLR4 agonists- Monophosphoryl lipid A[MPL]
TLR5 agonists- flagellin
TLR7/8 agonists- ssRNA
TLR9 agonists- CpG ODN
NOD agonists- Muramyl dipeptide](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-18-320.jpg)



![MUCOSAL ADJUVANTS
First immunization through mucosal surface was accomplished with
attenuated poliovirus in 1962.
Other mucosal vaccines based on Salmonella typhi, Vibrio cholerae,
rotavirus, and influenza virus were developed.
Administration by mucosal route has some advantages as needle free
delivery, lower costs, few adverse effects, and induction of local mucosal
immunity.
The most promising adjuvants for mucosal immunization are bacterial
toxins extacted from Escherichia coli[heat labile toxin] and vibrio
cholerae[cholera toxin], TLR agonists and novel small molecules[alpha-
galactosylceramide, chitosan].](https://image.slidesharecdn.com/adjuvants-1-221122151059-88af0583/85/ADJUVANTS-1-pptx-22-320.jpg)


