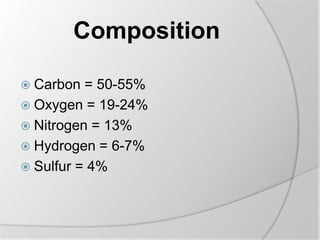
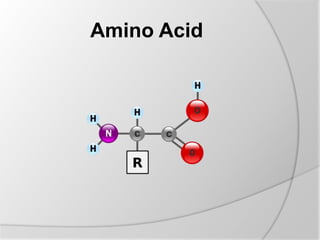
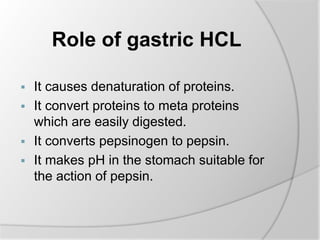
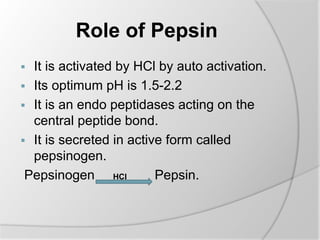
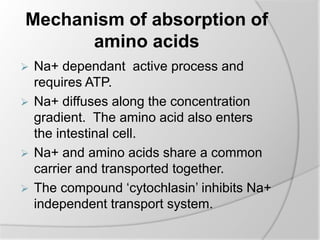
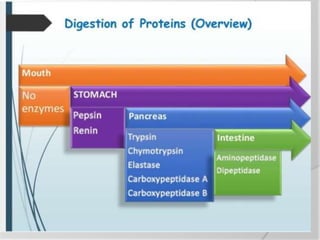
The document discusses the absorption and digestion of proteins, detailing their structure, function, and the biochemical processes involved in their breakdown within the digestive system. Proteins, primarily composed of amino acids, undergo digestion starting in the stomach and continuing in the pancreas and intestines, where various enzymes like pepsin and trypsin play a crucial role. Amino acid absorption occurs actively in the small intestine through transport systems that require energy, and is essential for various cellular functions.