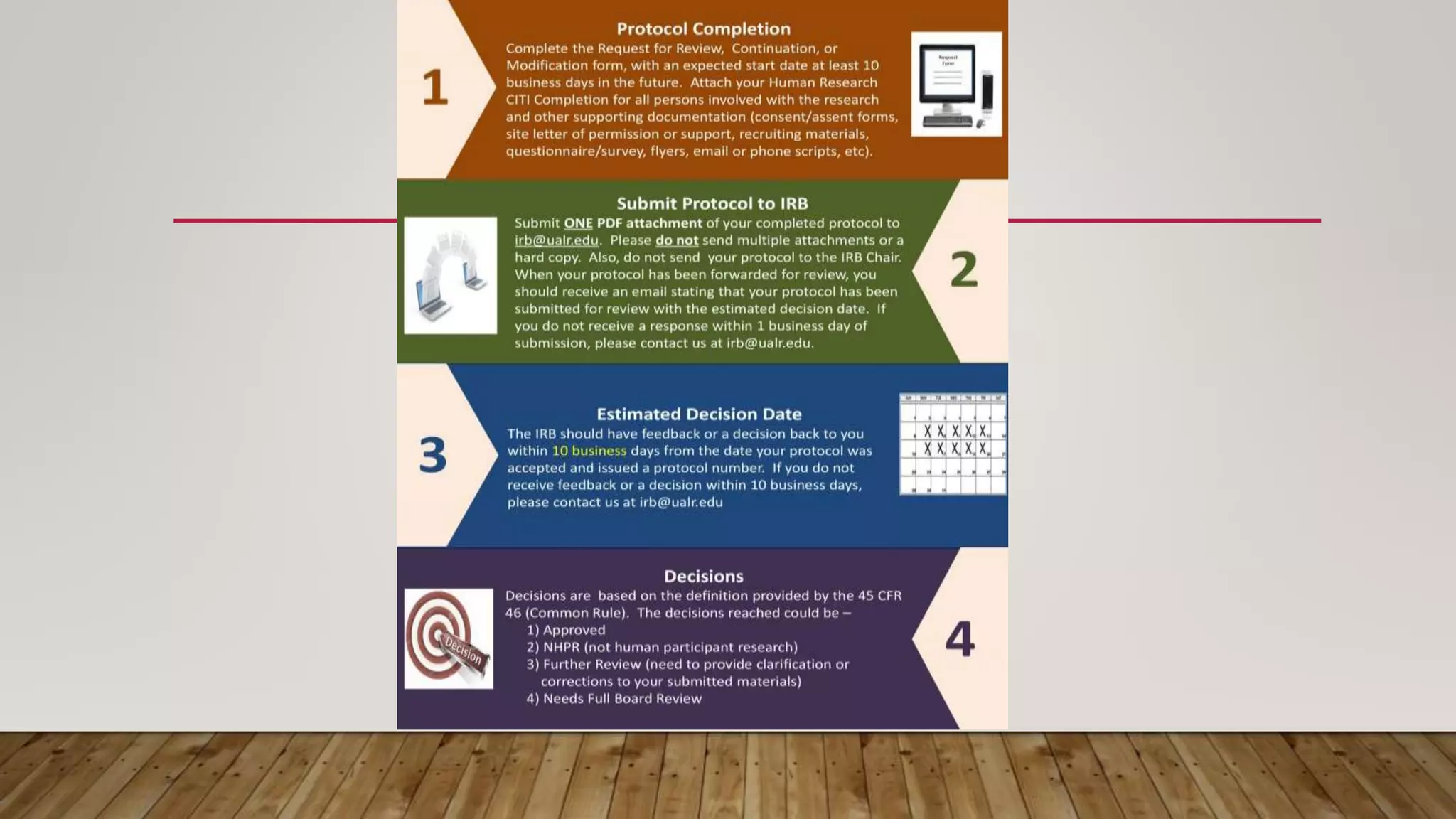
This document defines and describes the role and responsibilities of an Institutional Review Board (IRB). The key points are:
1) An IRB is responsible for reviewing and approving research involving human subjects to ensure ethical standards are met and subjects' rights and safety are protected.
2) IRBs must have diverse and qualified membership, including scientific and non-scientific experts, to conduct in-depth reviews of research protocols.
3) The IRB review process involves evaluating research protocols, consent forms, recruitment procedures and investigators to ensure all applicable regulations and ethical guidelines are followed.