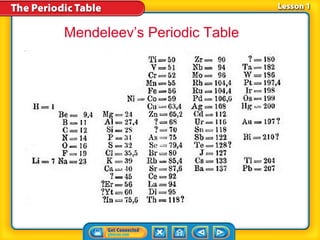
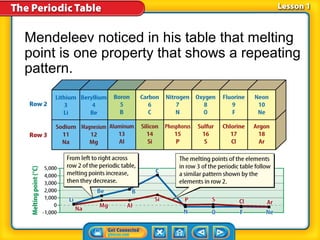
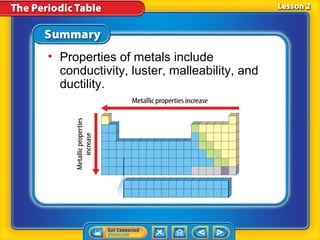
The periodic table is a chart that arranges the elements in rows and columns according to their physical and chemical properties. Elements in the same group have similar properties, and an element's location on the periodic table provides information about its properties. Metals are generally located on the left side and middle of the periodic table and have properties such as conductivity and luster, while nonmetals are usually gases or brittle solids found on the right side. Metalloids exhibit some properties of both metals and nonmetals.