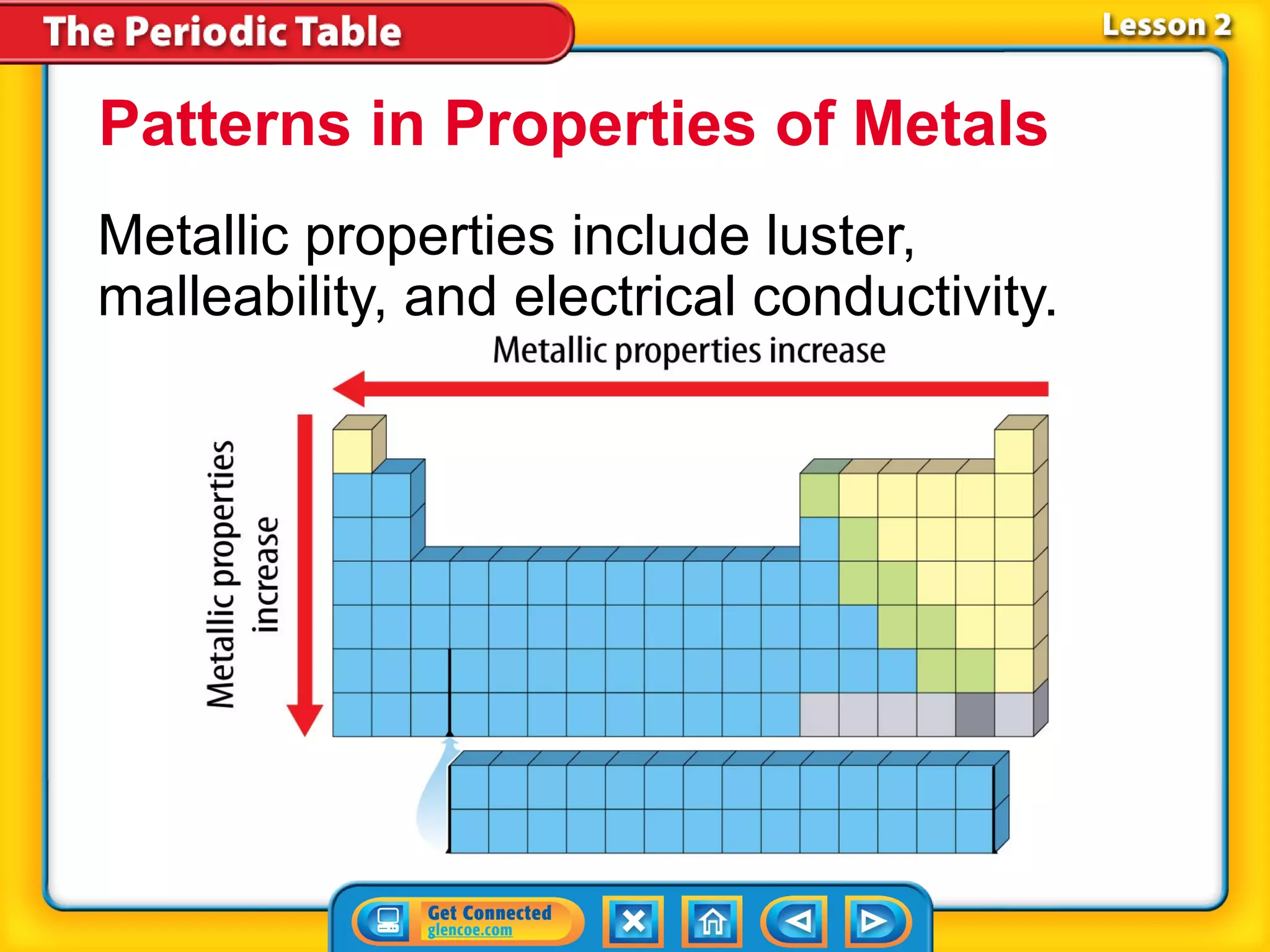
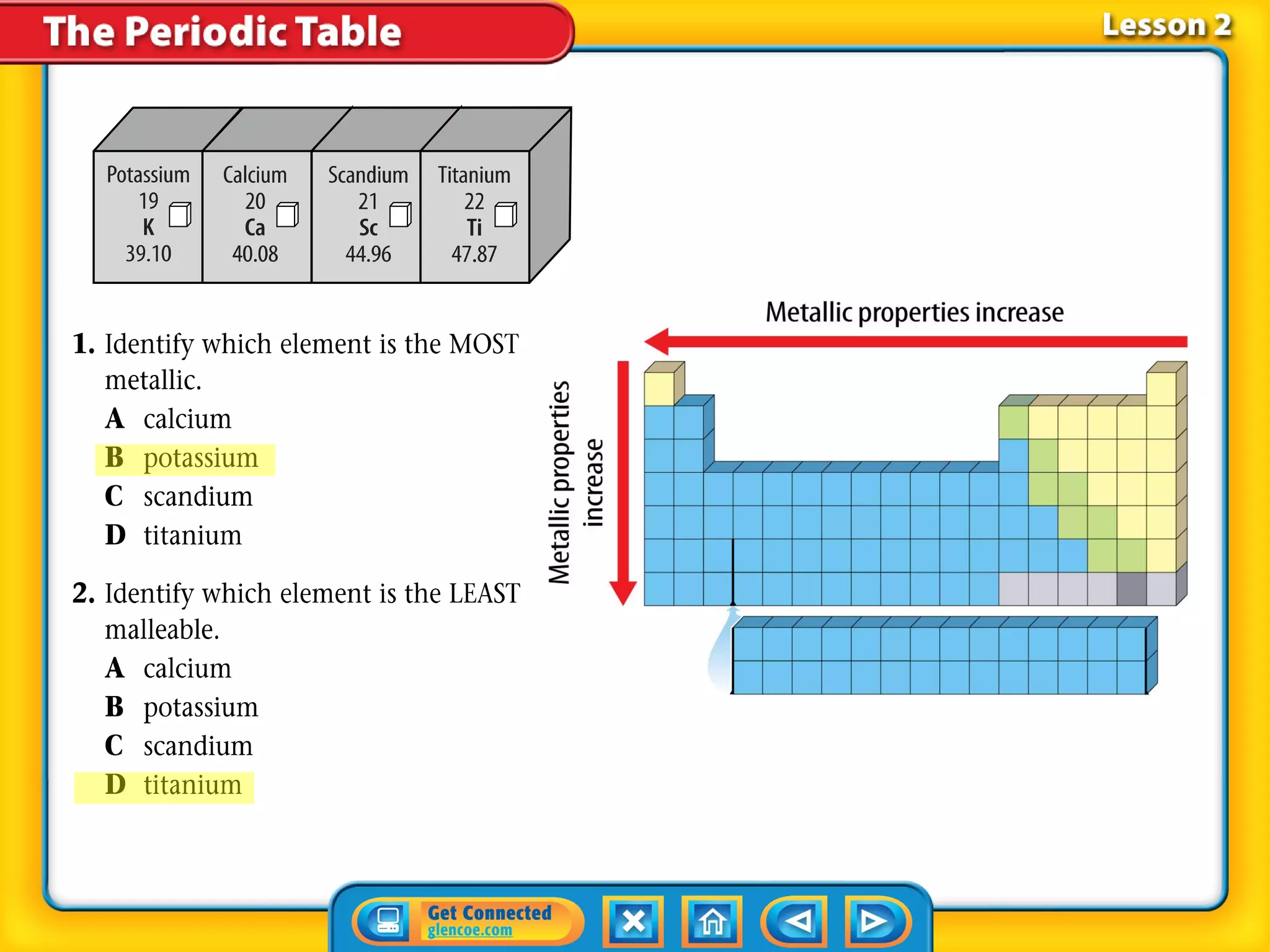
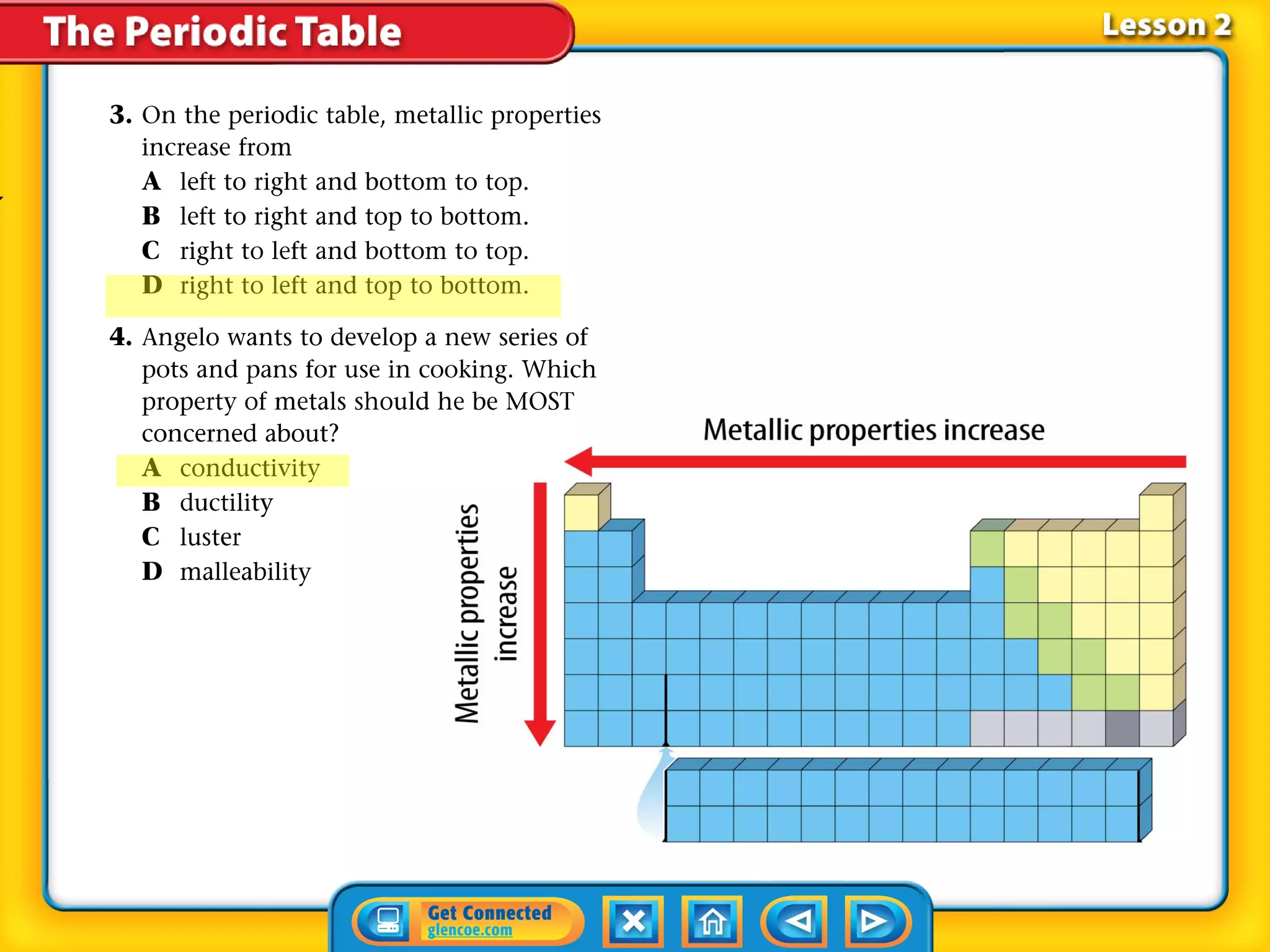
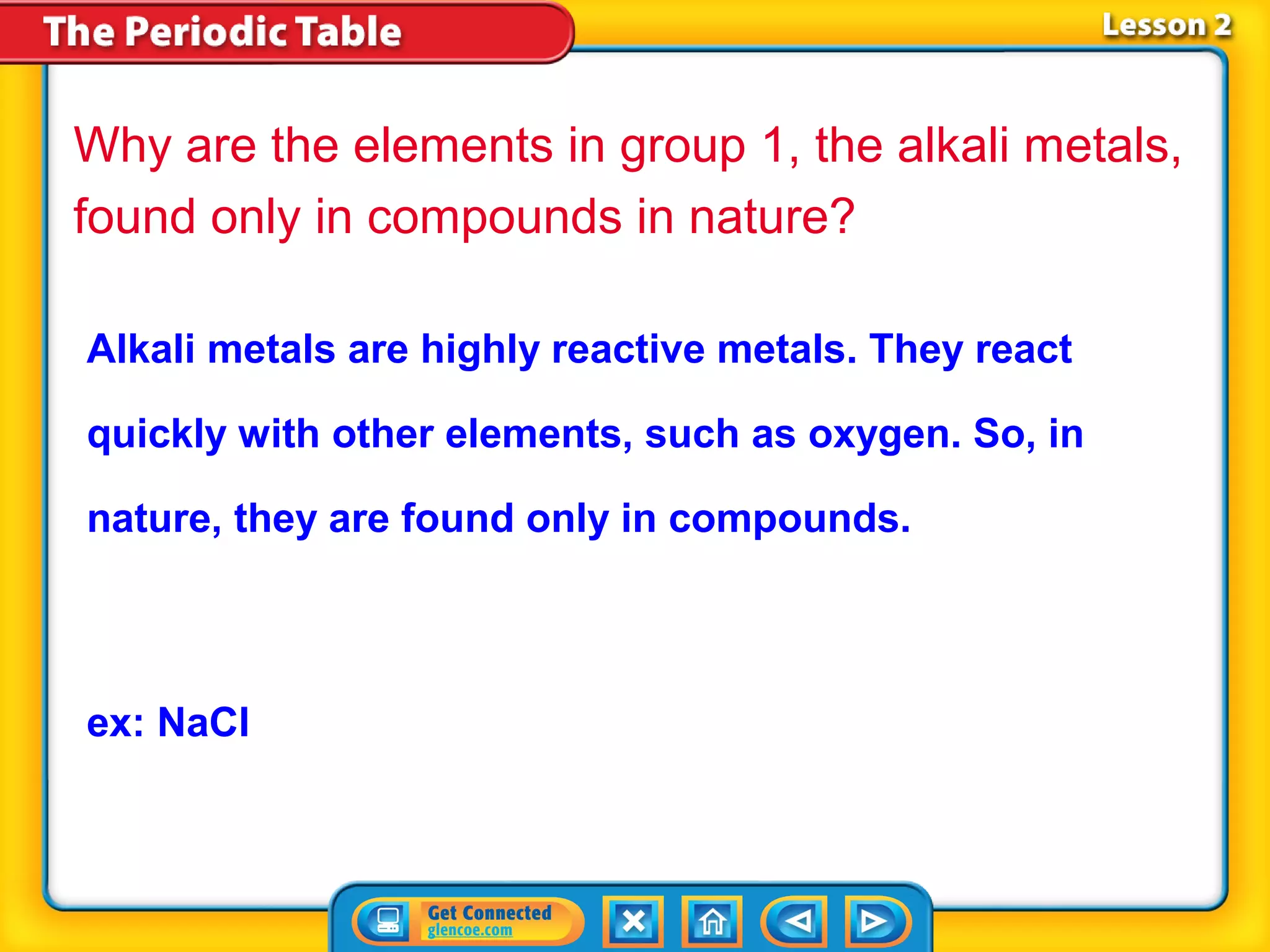
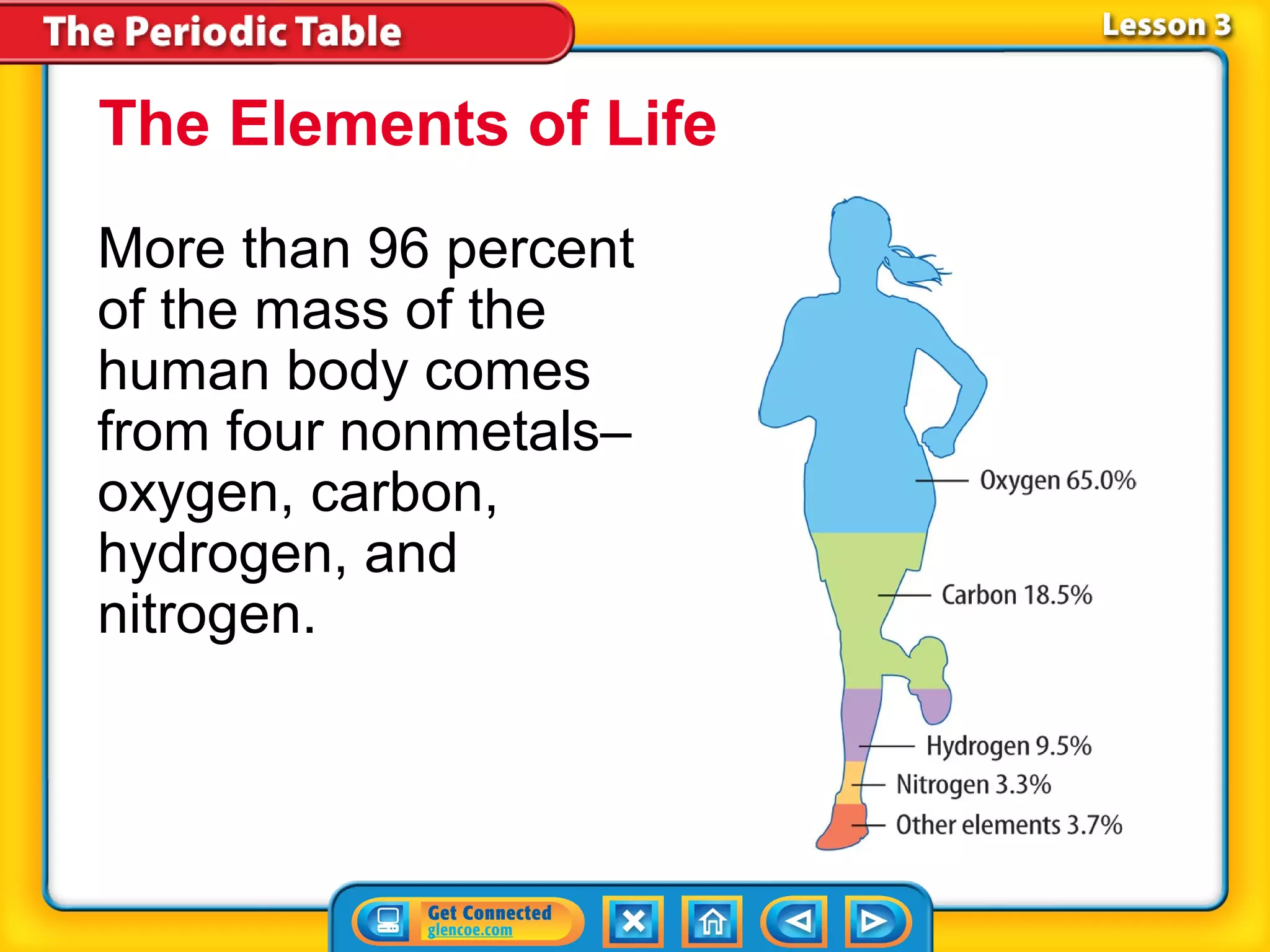
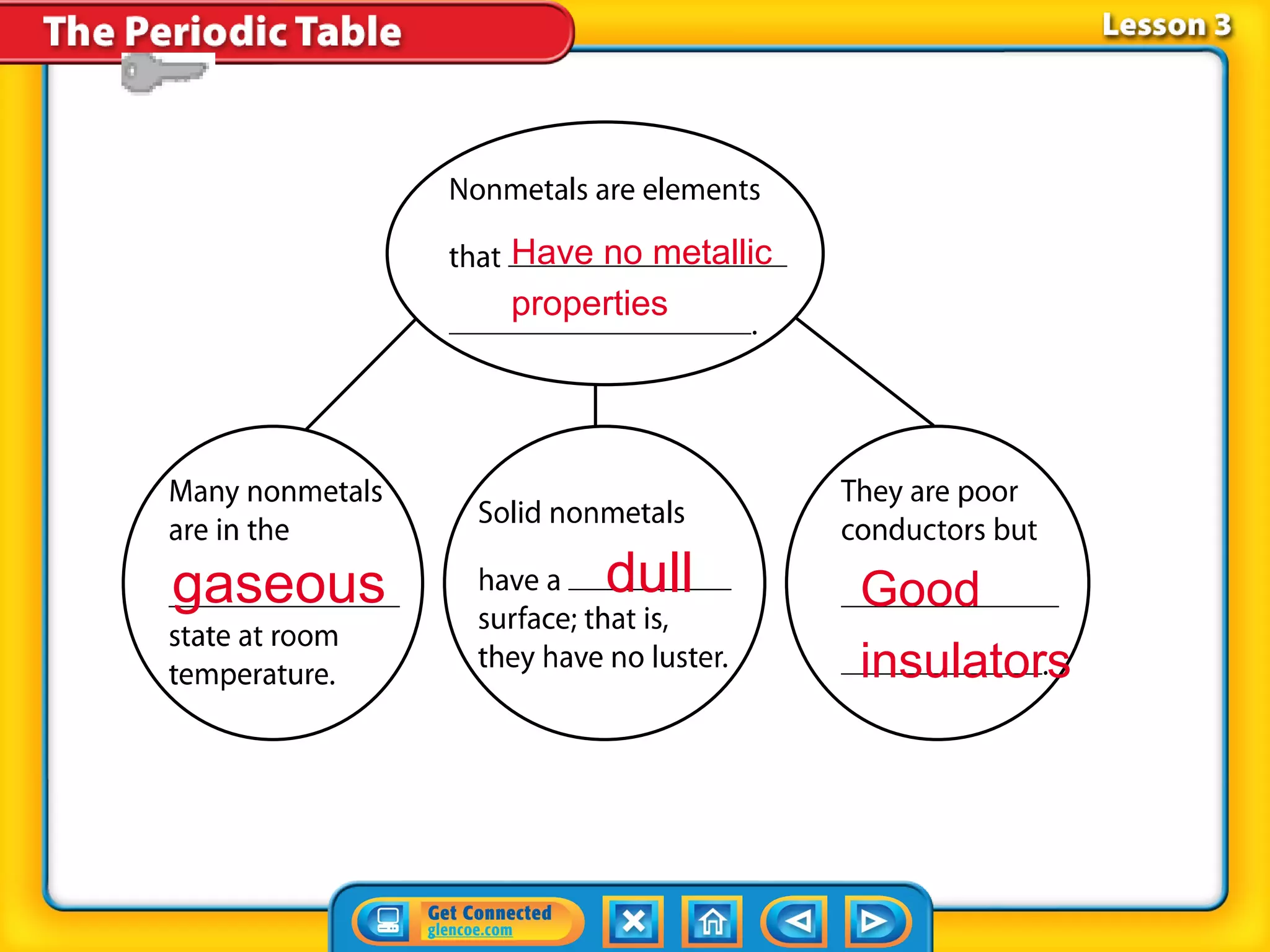
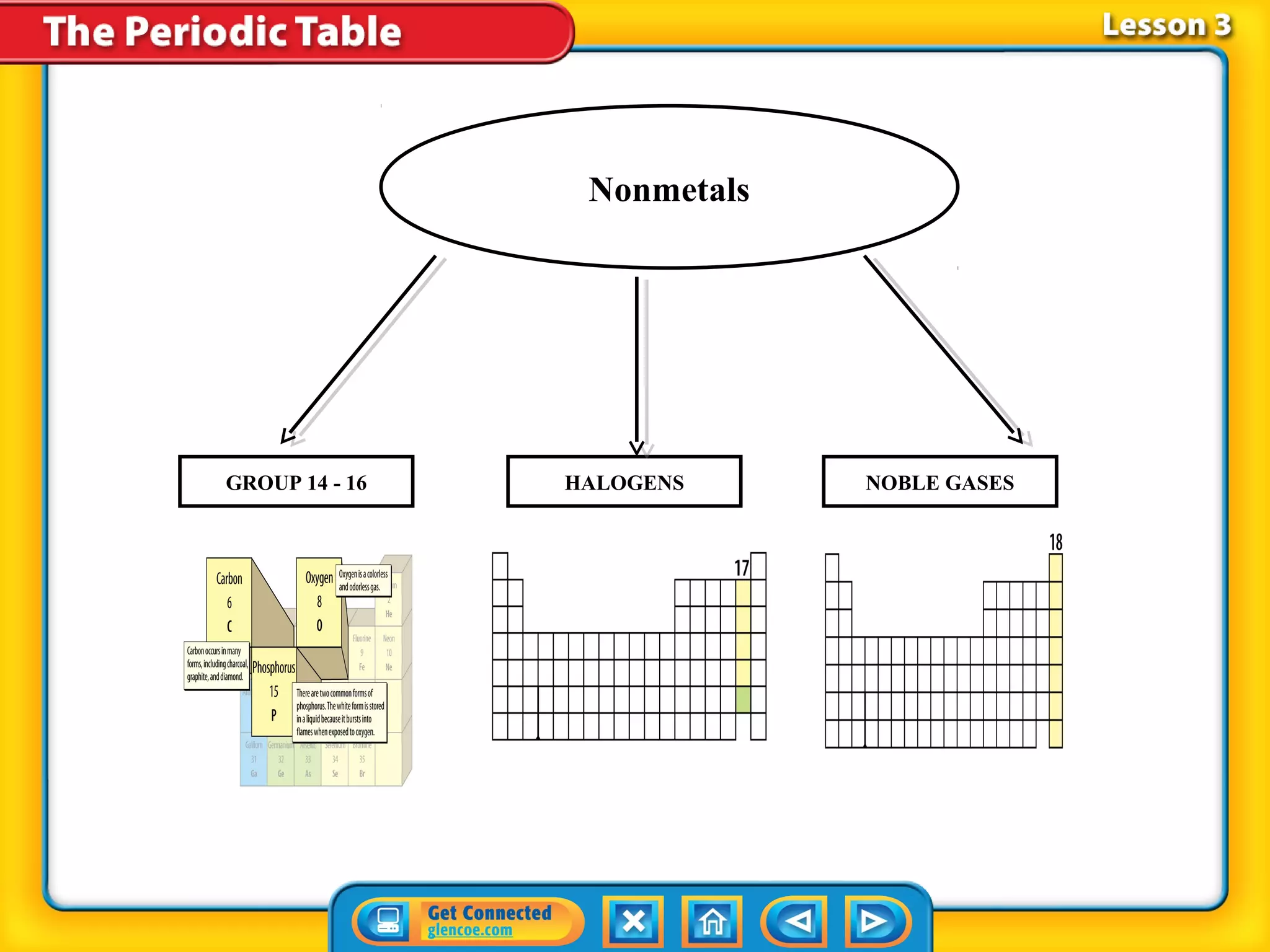
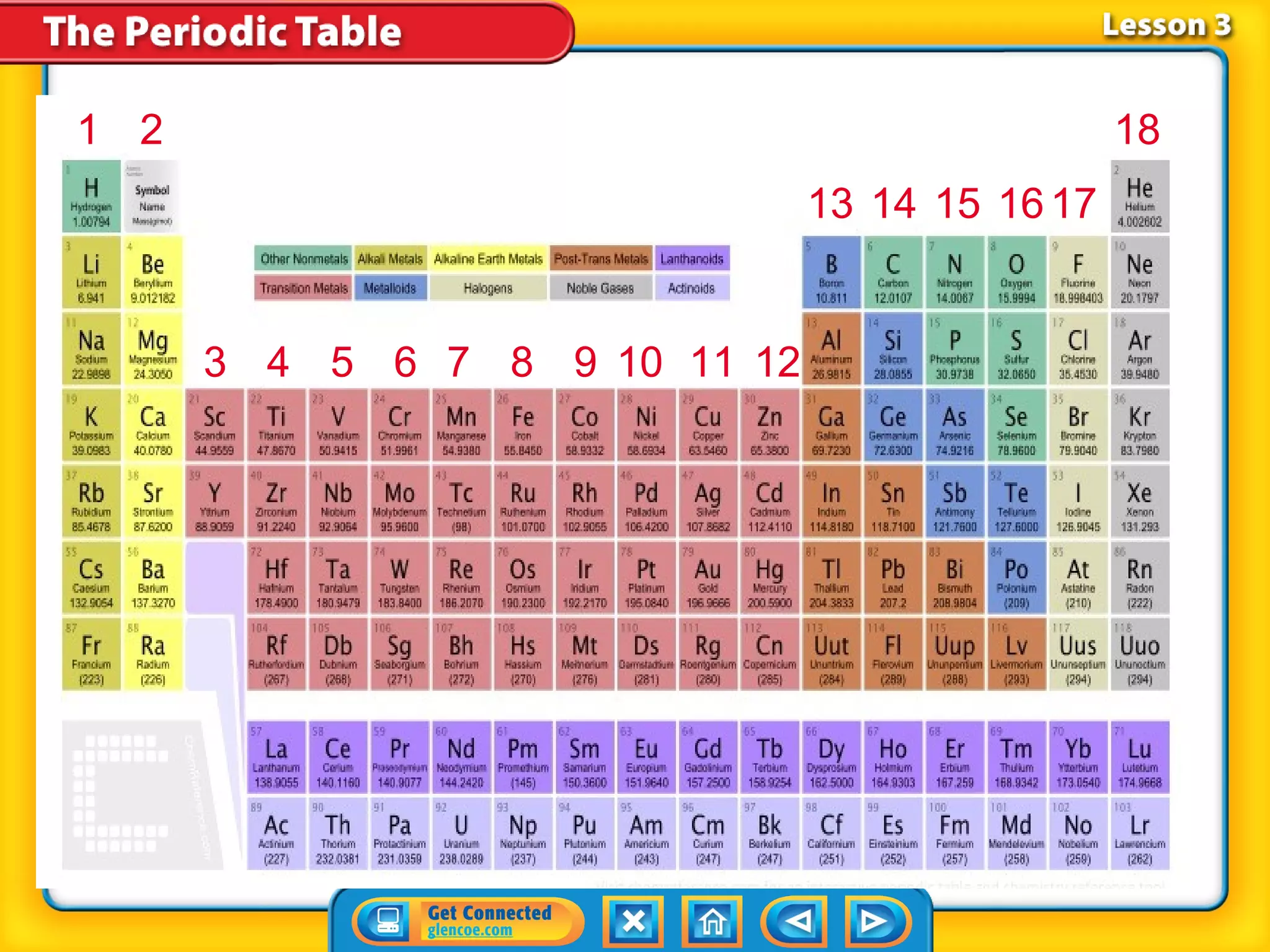
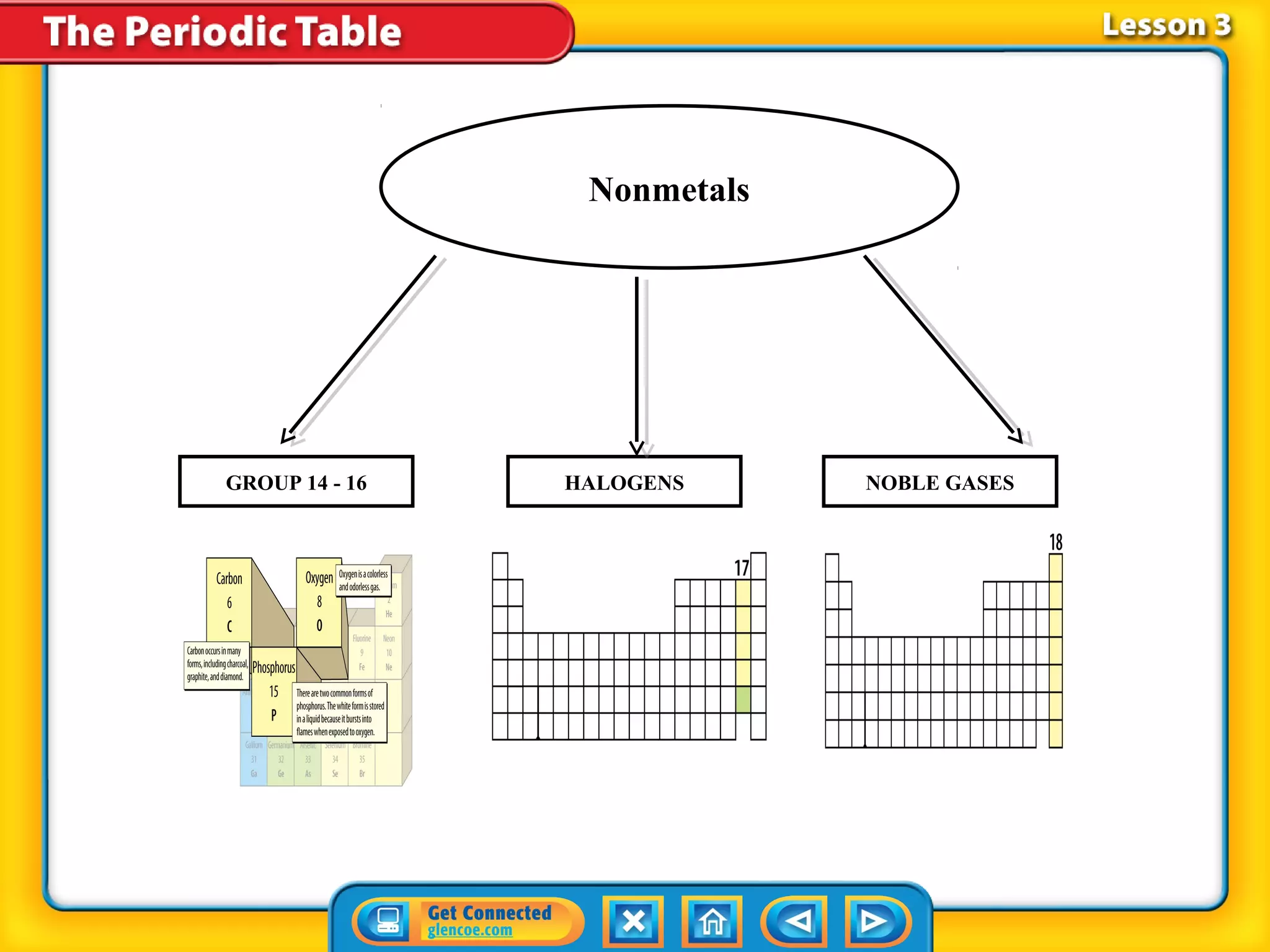
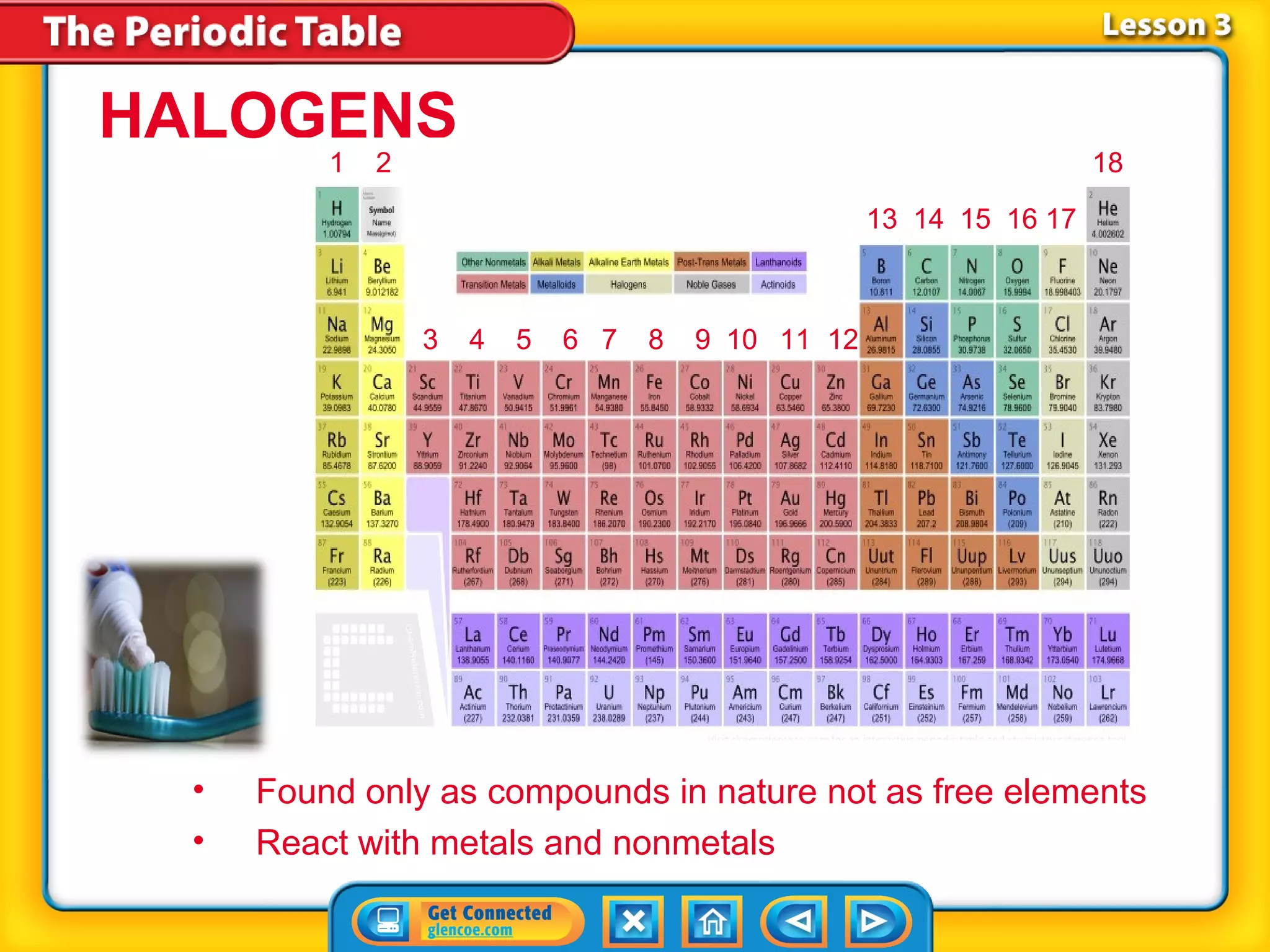
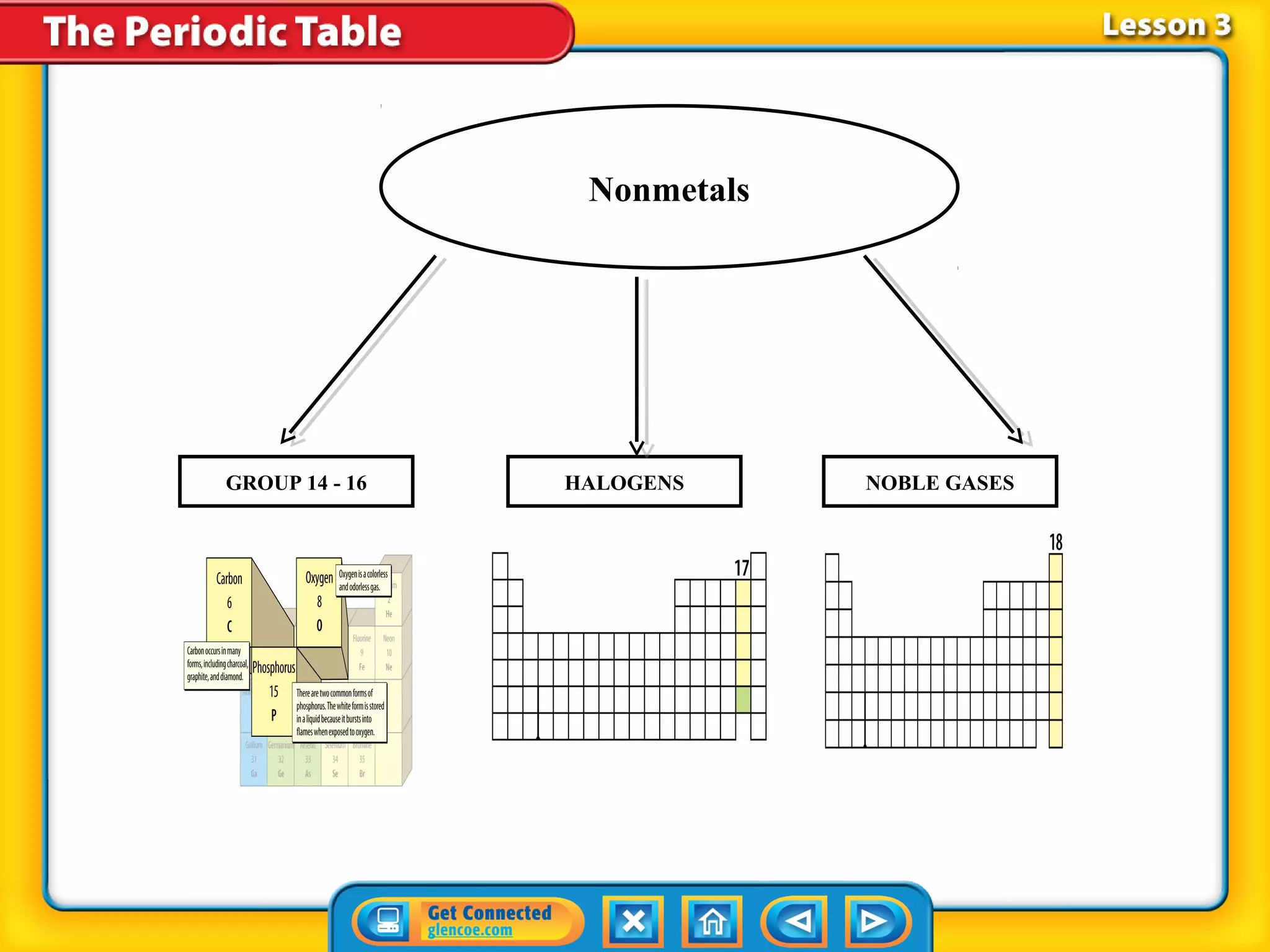
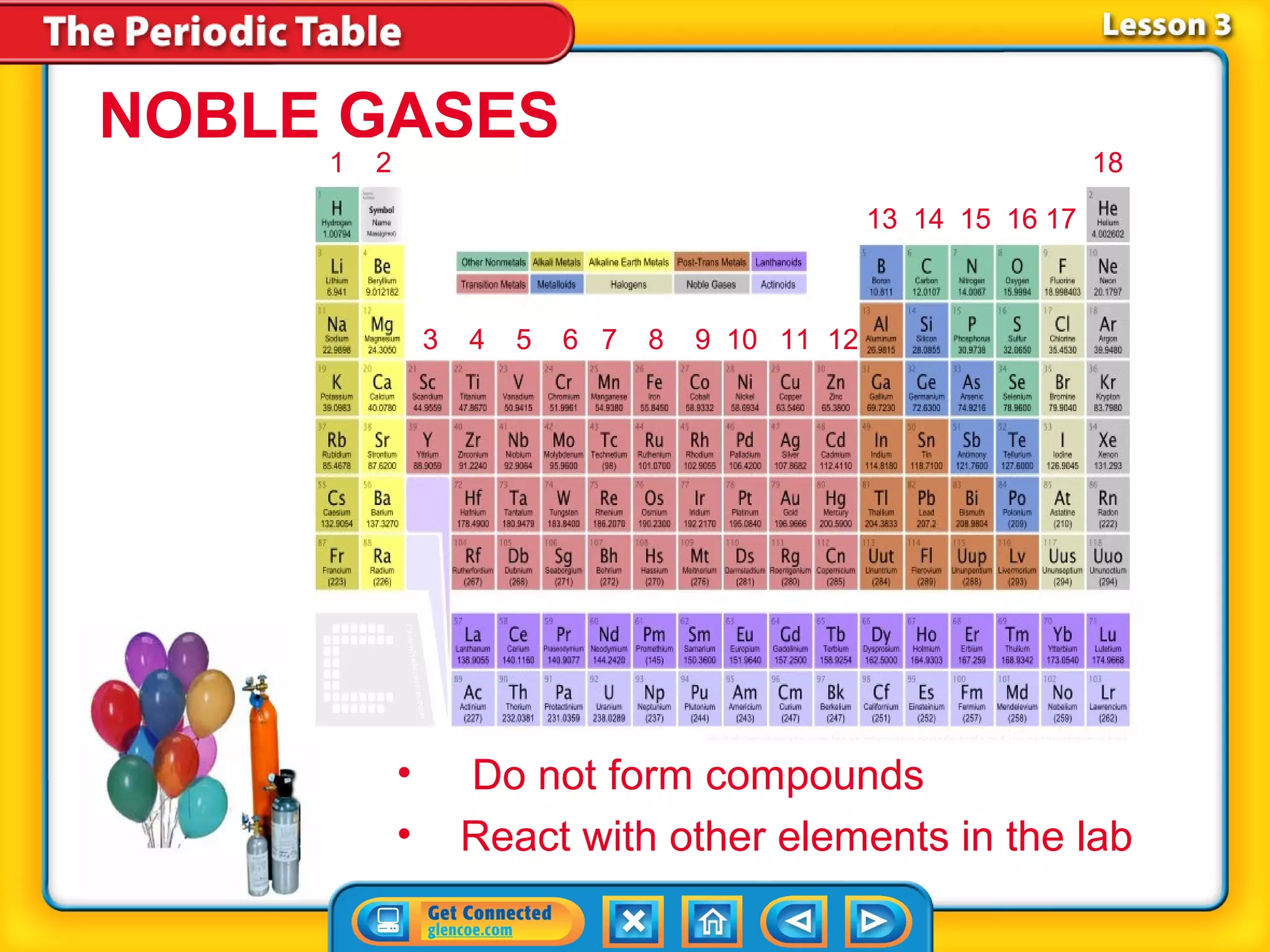
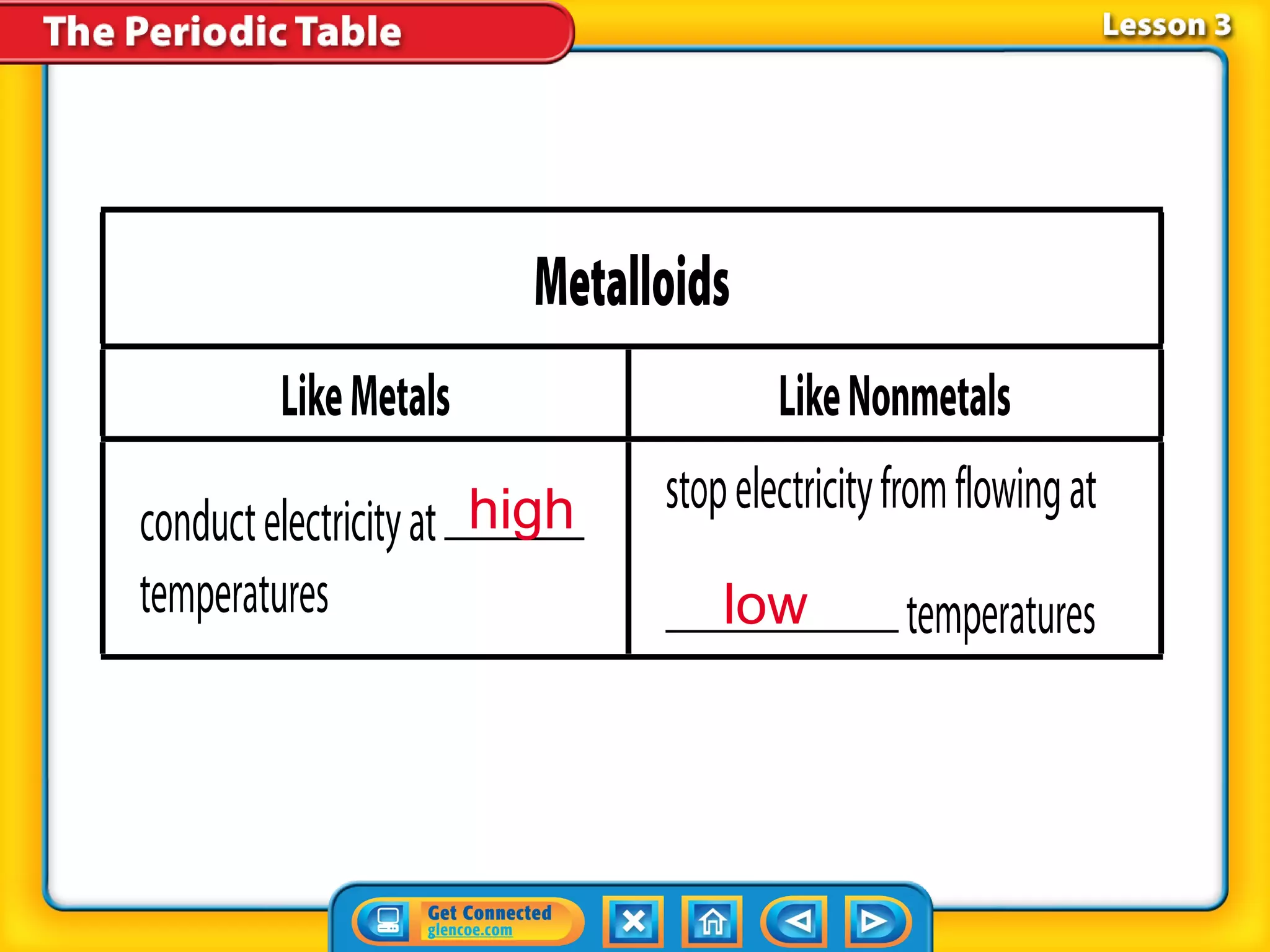
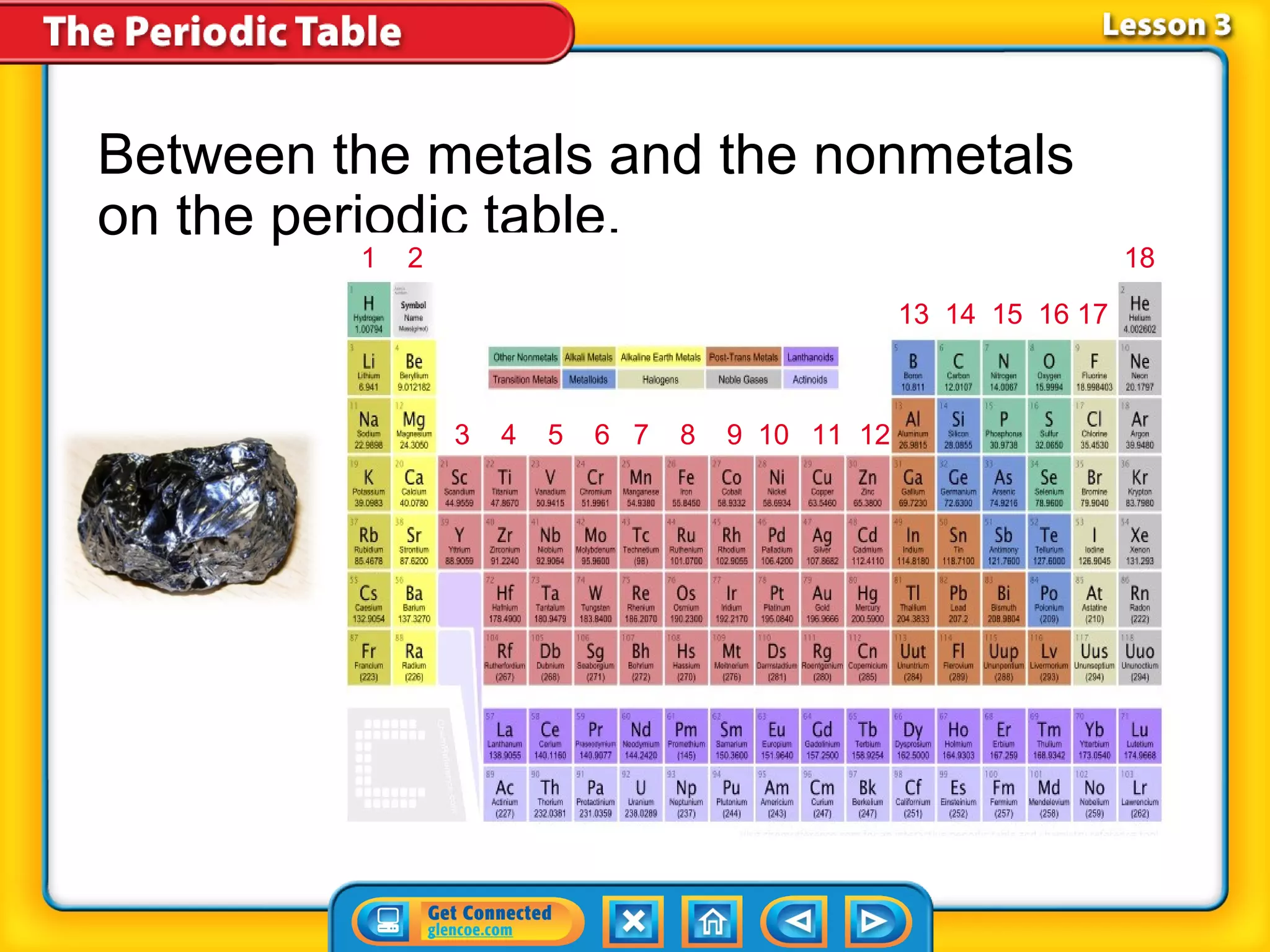
The document discusses properties of metals and nonmetals. It notes that alkali metals are highly reactive and found only in compounds in nature because they react quickly with oxygen. It also states that more than 96% of the human body's mass comes from four nonmetals: oxygen, carbon, hydrogen, and nitrogen. Nonmetals and metalloids are located between metals and nonmetals on the periodic table, have no metallic properties, and are good insulators.