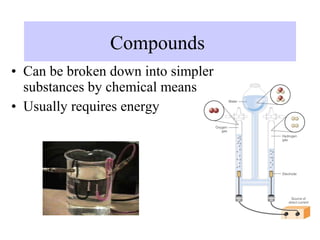
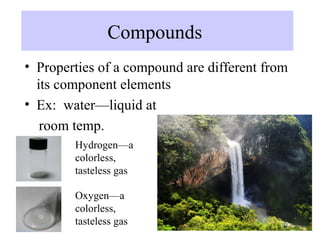
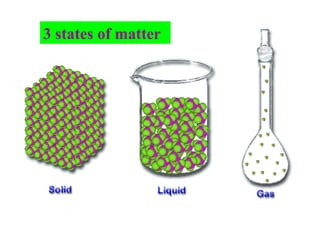
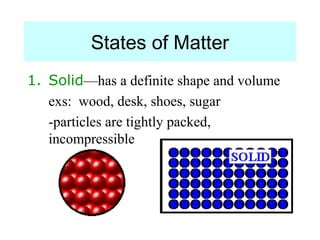
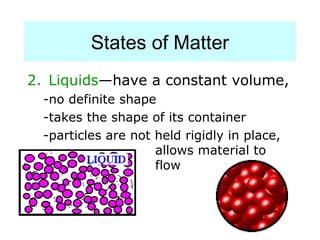
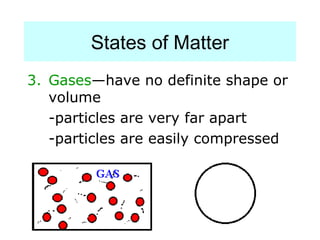
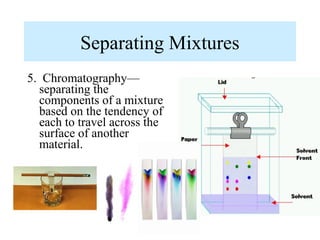
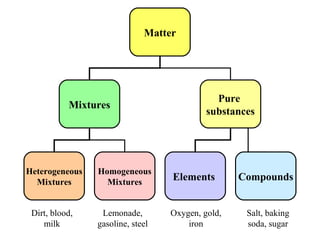
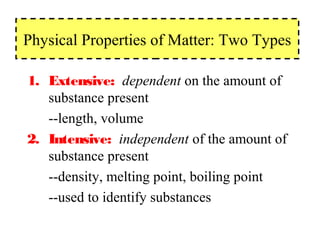
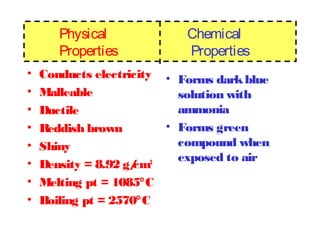
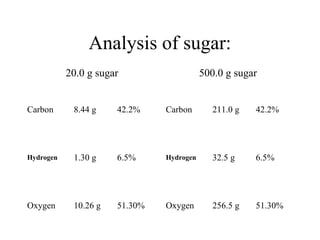
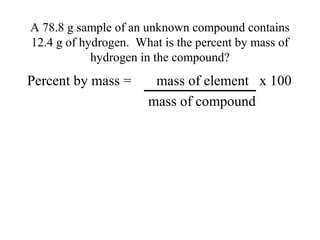
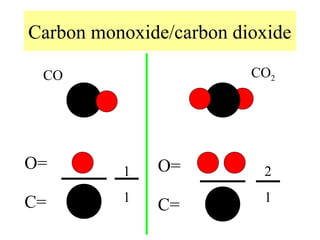
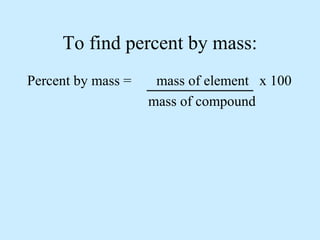The document discusses the properties and types of matter, including the three states of matter (solid, liquid, gas), mixtures and their separation, physical and chemical properties, physical and chemical changes, and the laws of conservation of mass, definite proportions, and multiple proportions as they relate to matter and chemical reactions. Elements are pure substances that cannot be broken down further, while compounds are combinations of two or more elements that have properties different from their component elements. Matter is anything that has mass and takes up space.