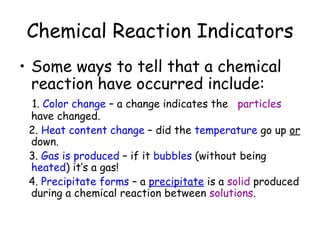
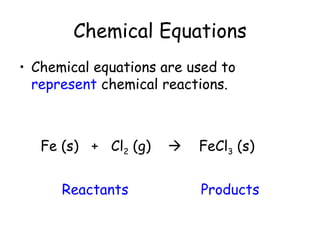
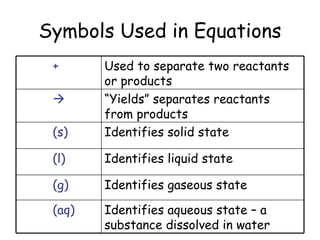
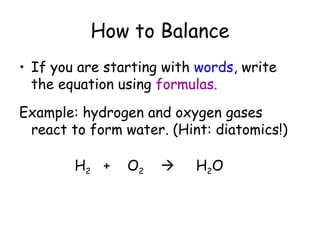
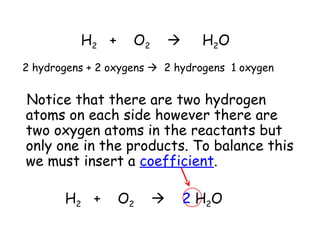
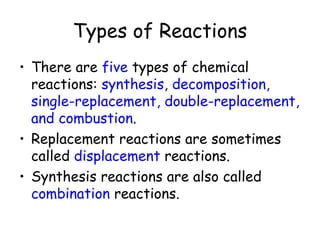
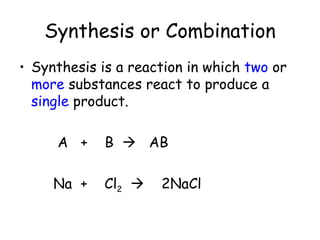
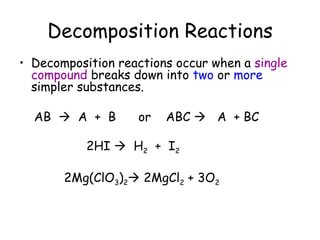
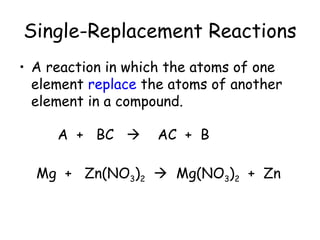
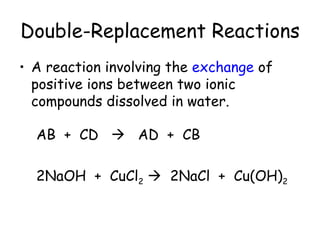
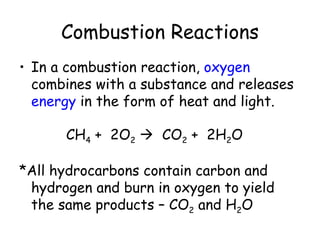
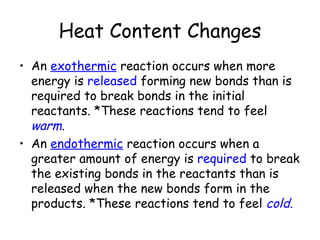
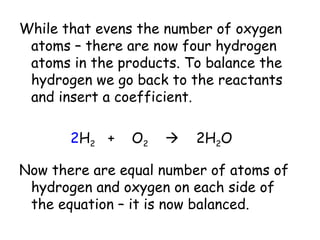A chemical reaction involves the rearrangement of atoms in one or more substances to form different substances. Chemical reactions can be identified by changes in color, temperature, production of gas, or formation of a precipitate. Chemical equations are used to represent reactions, with reactants on the left and products on the right. Equations must be balanced to ensure the same number and type of atoms are on both sides. The five main types of chemical reactions are synthesis, decomposition, single-replacement, double-replacement, and combustion.