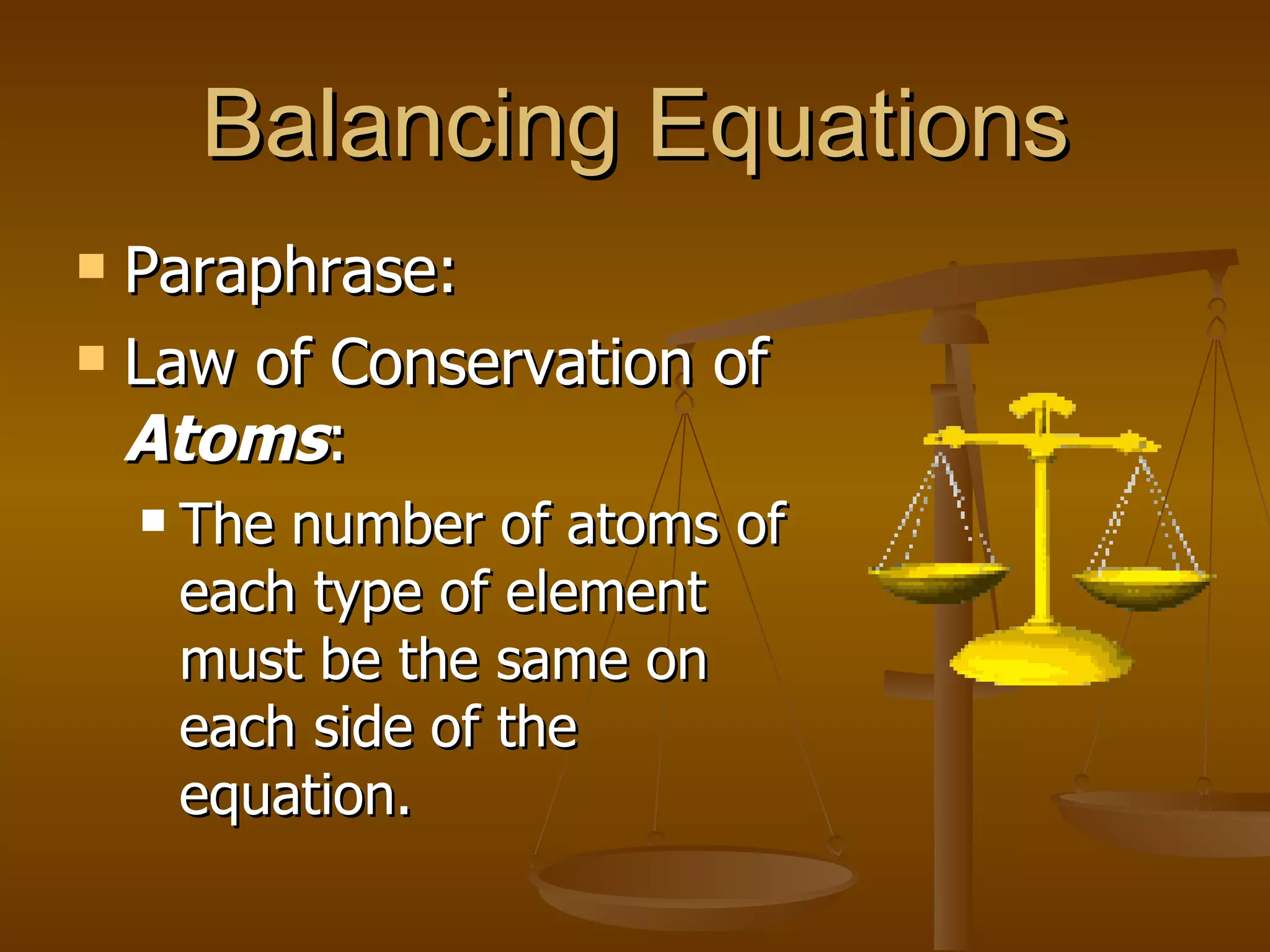
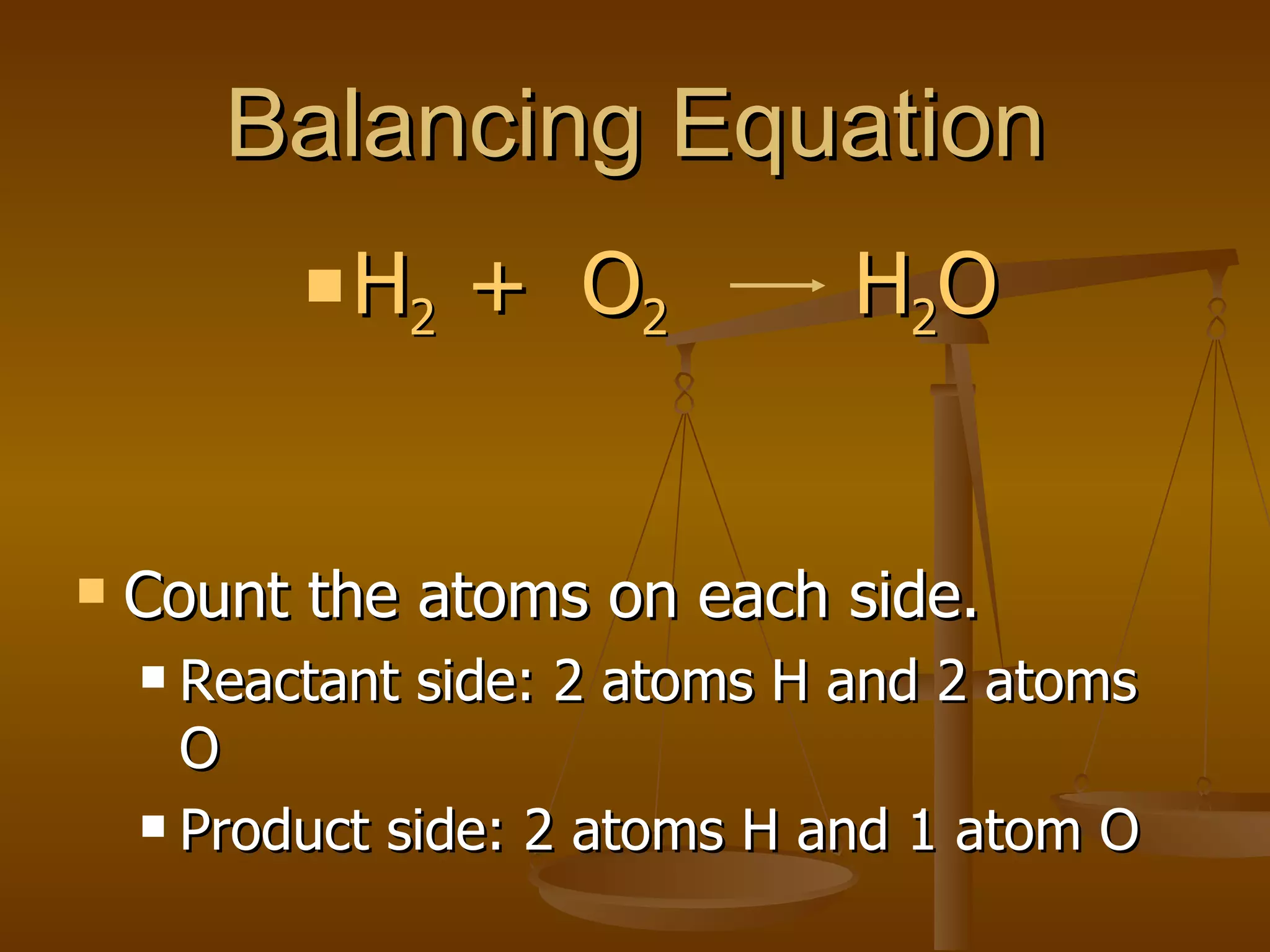
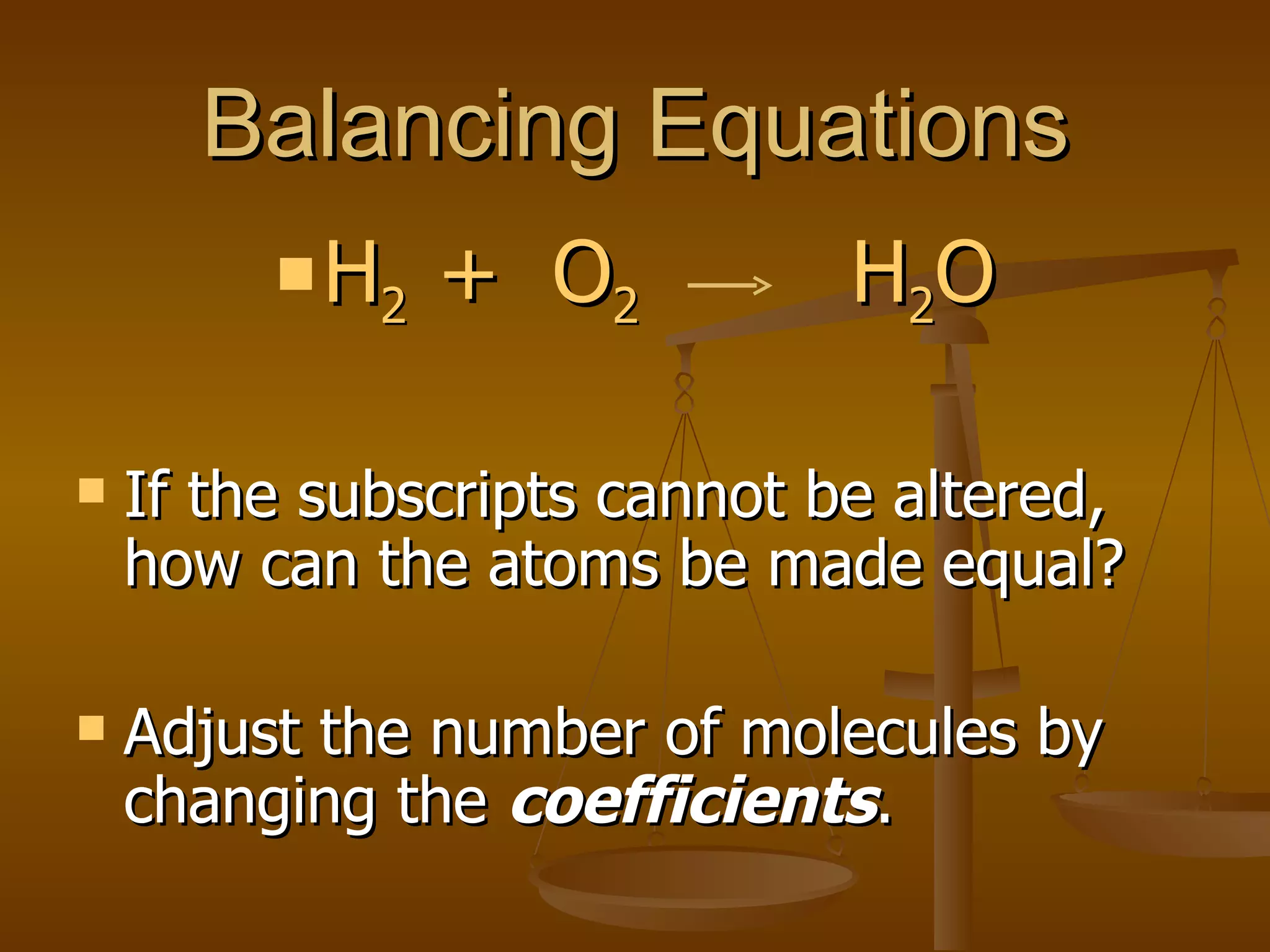
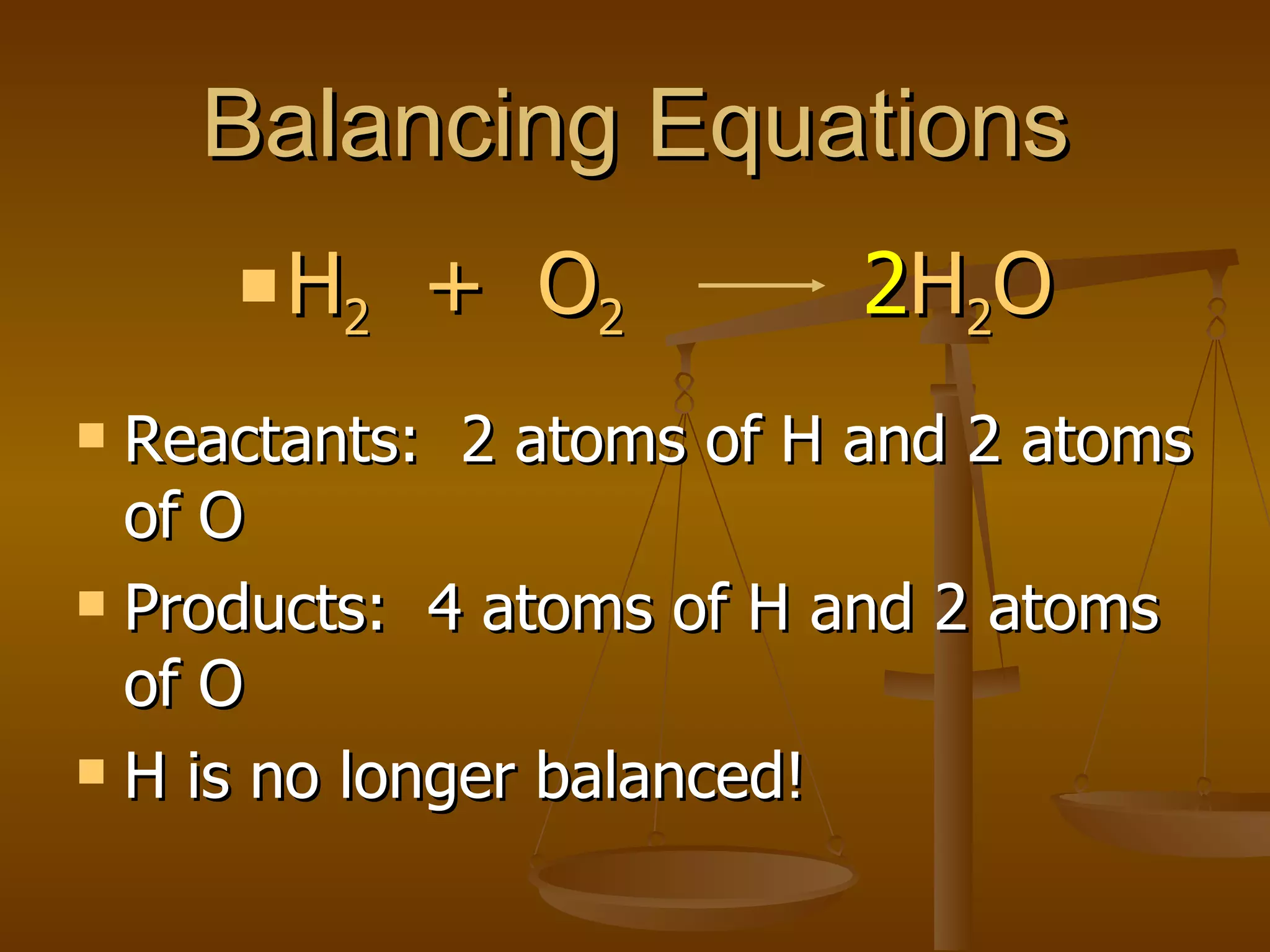
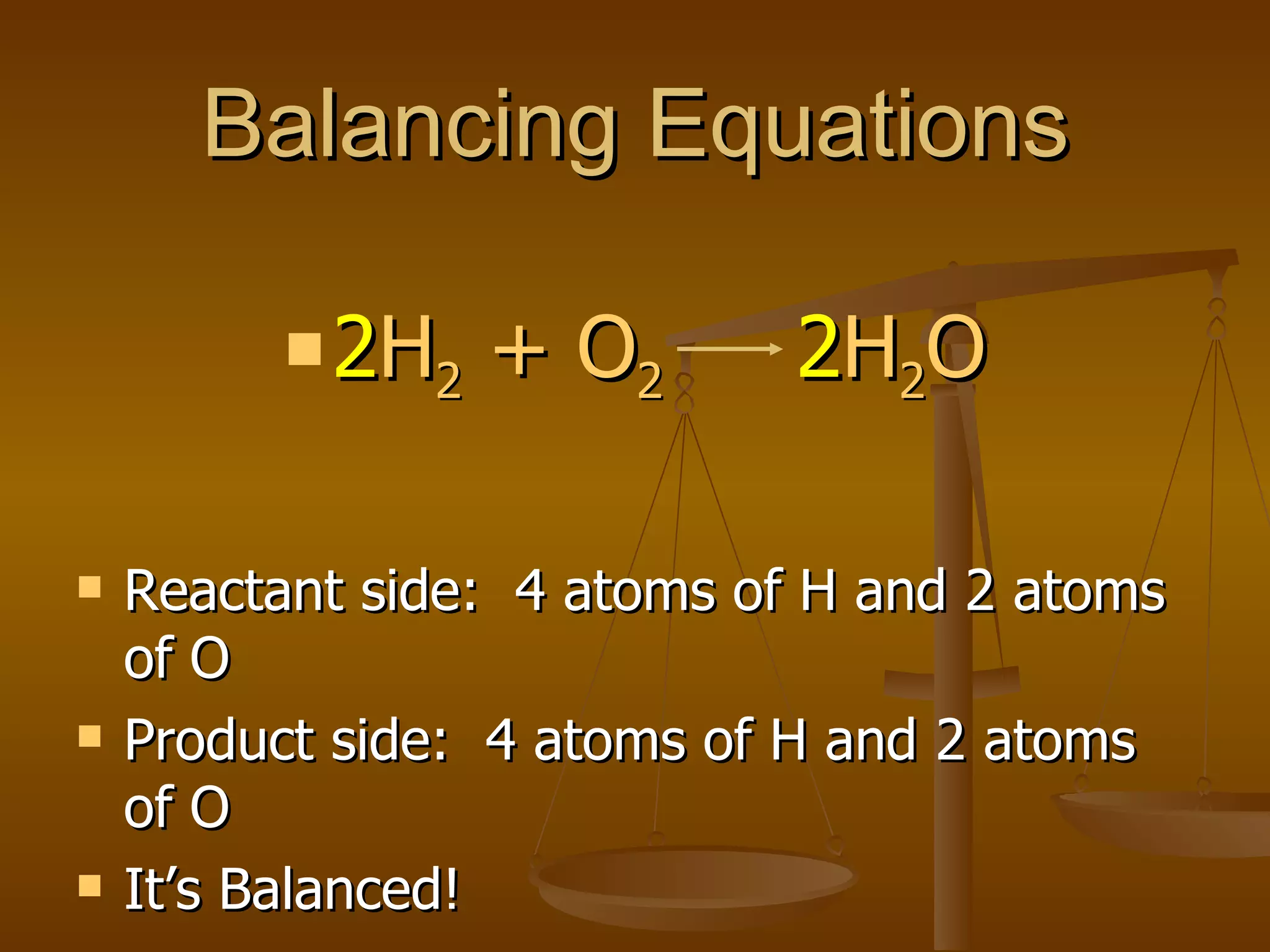
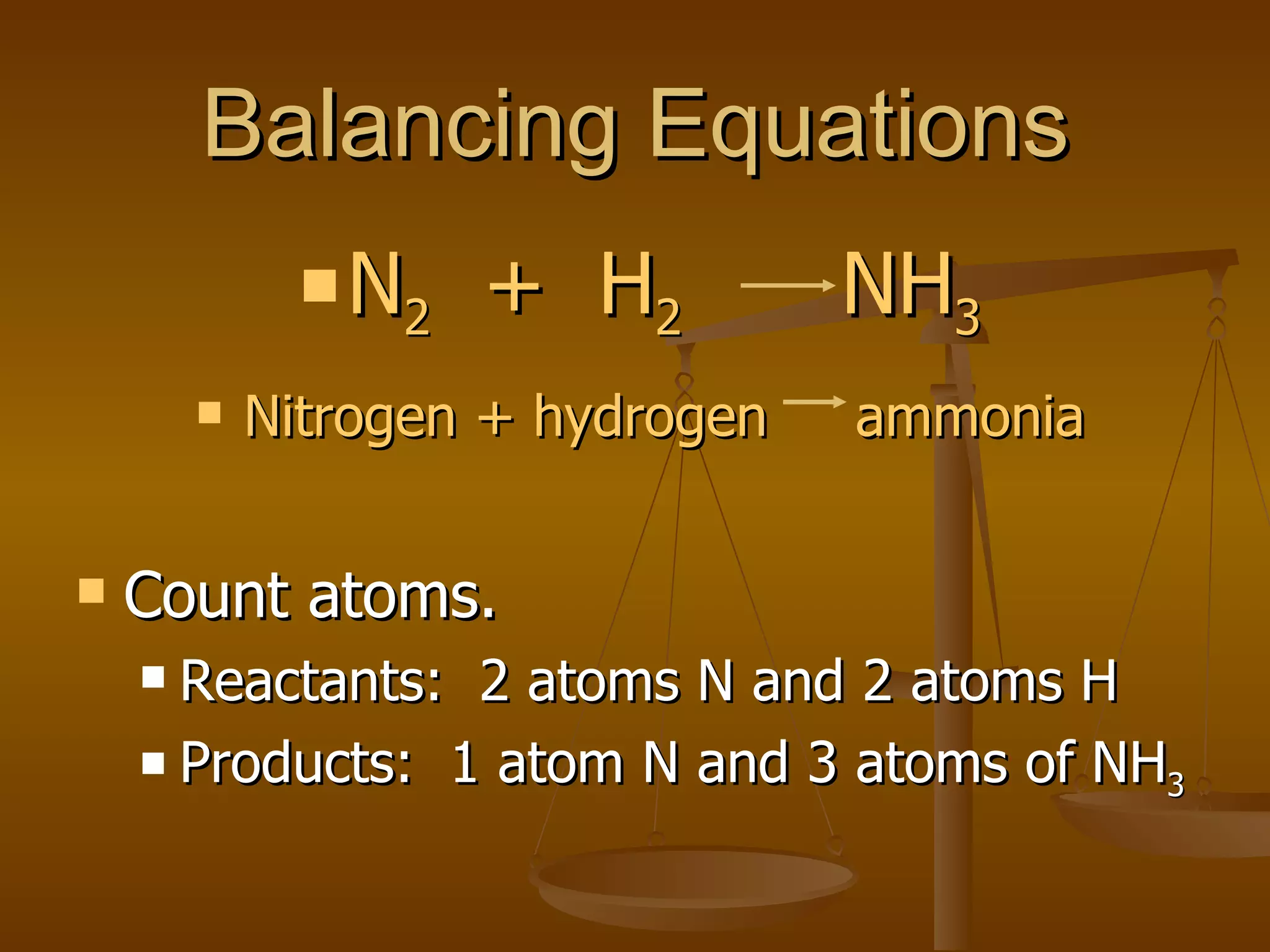
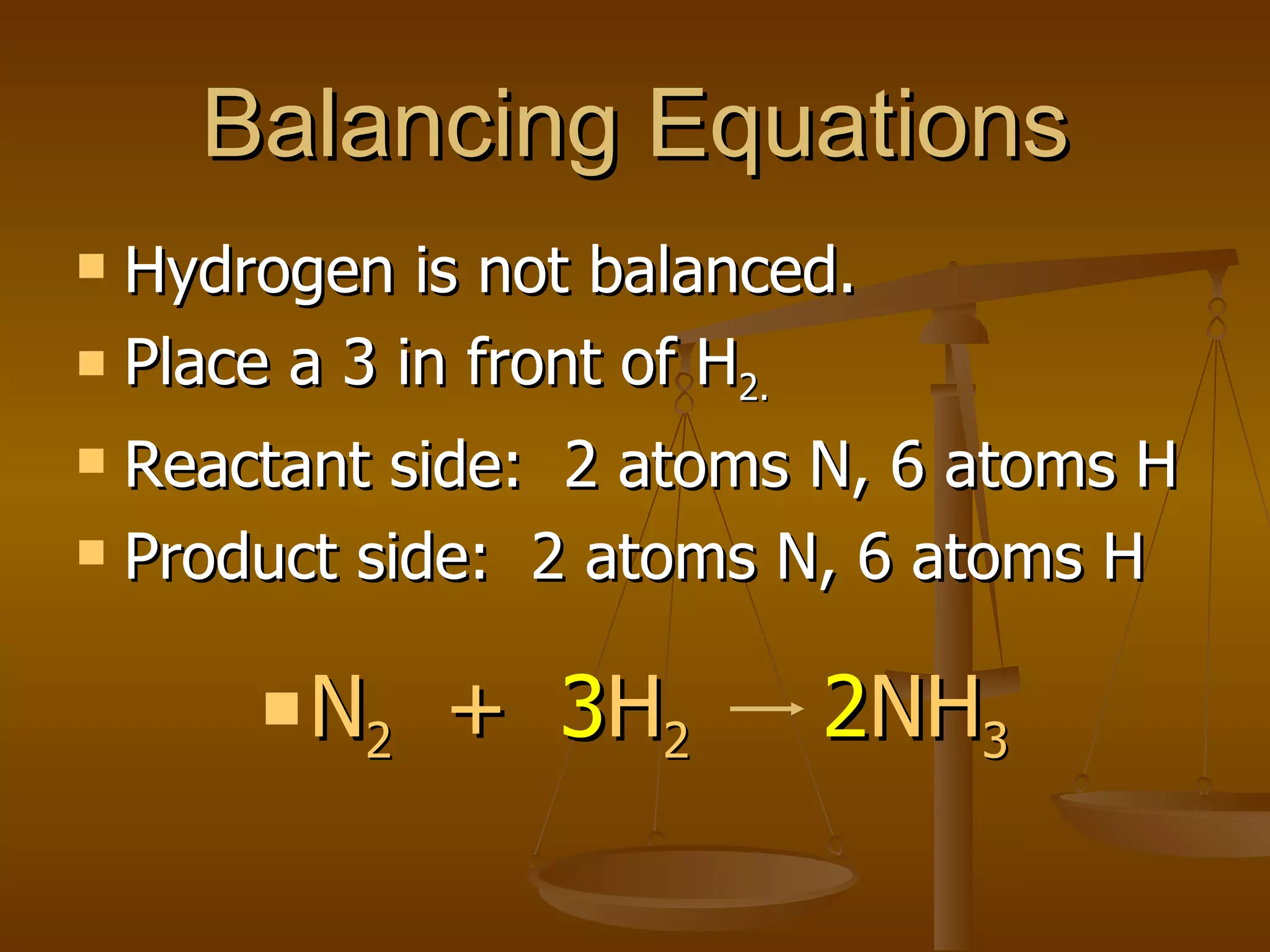
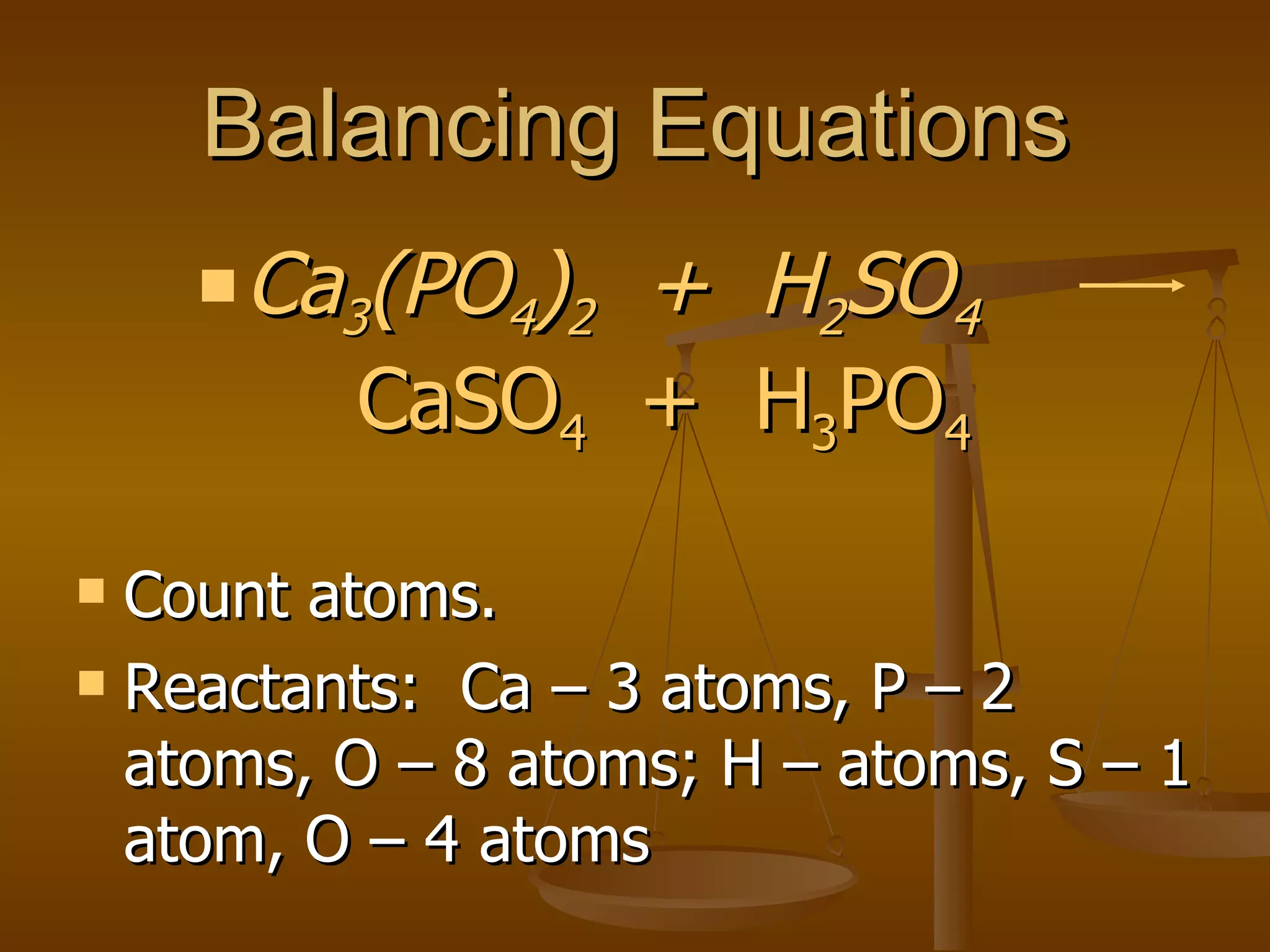
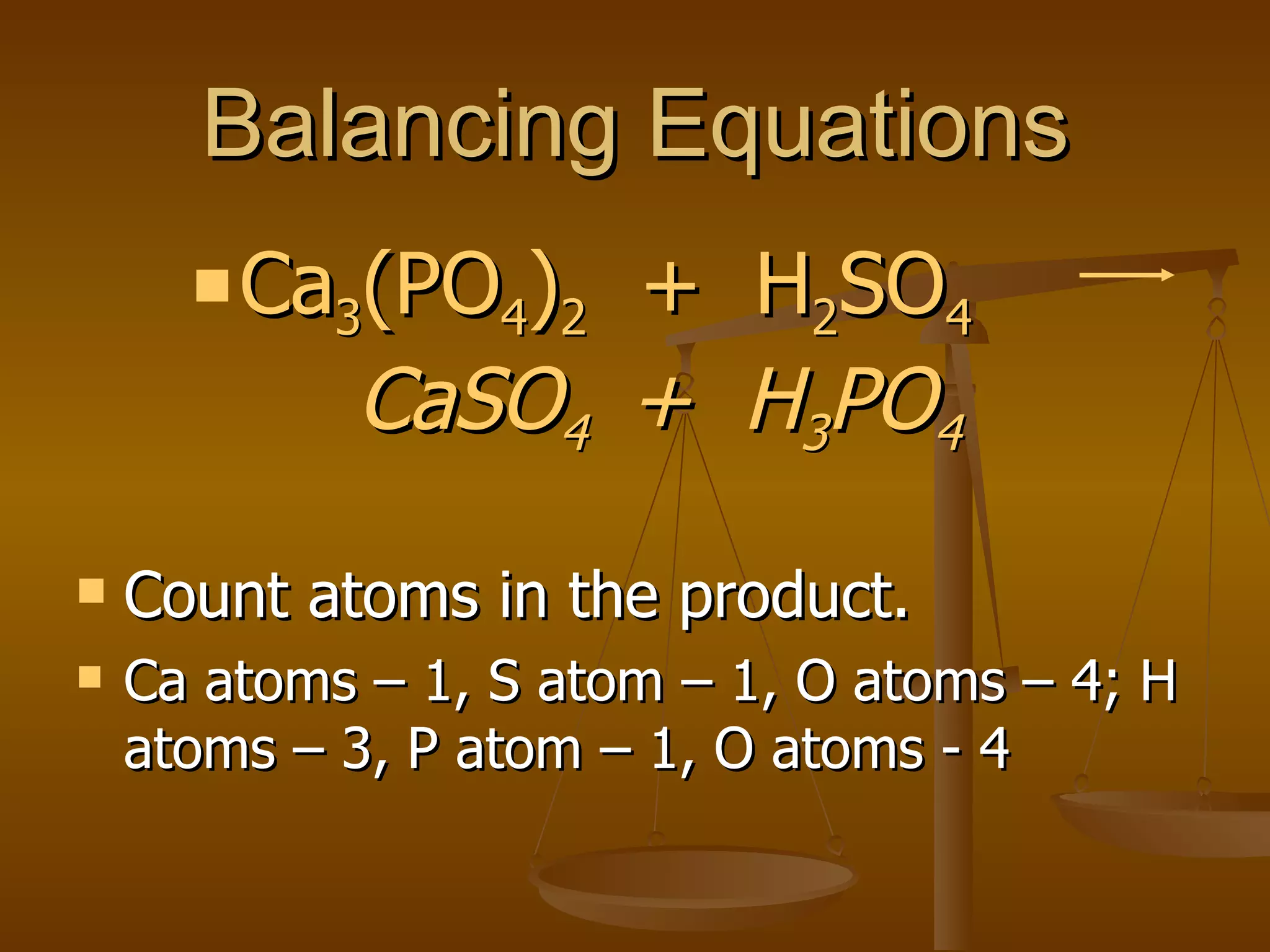
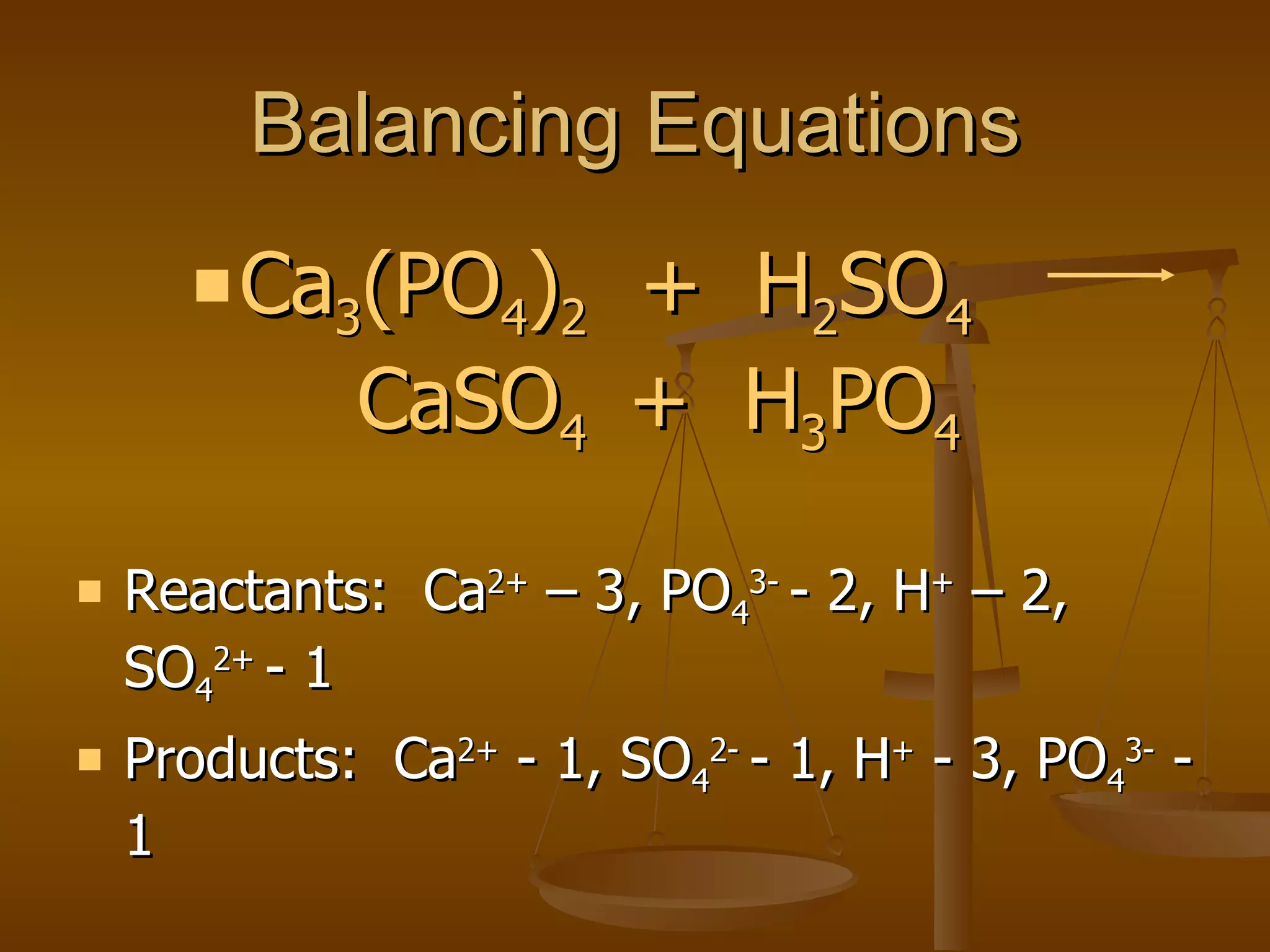
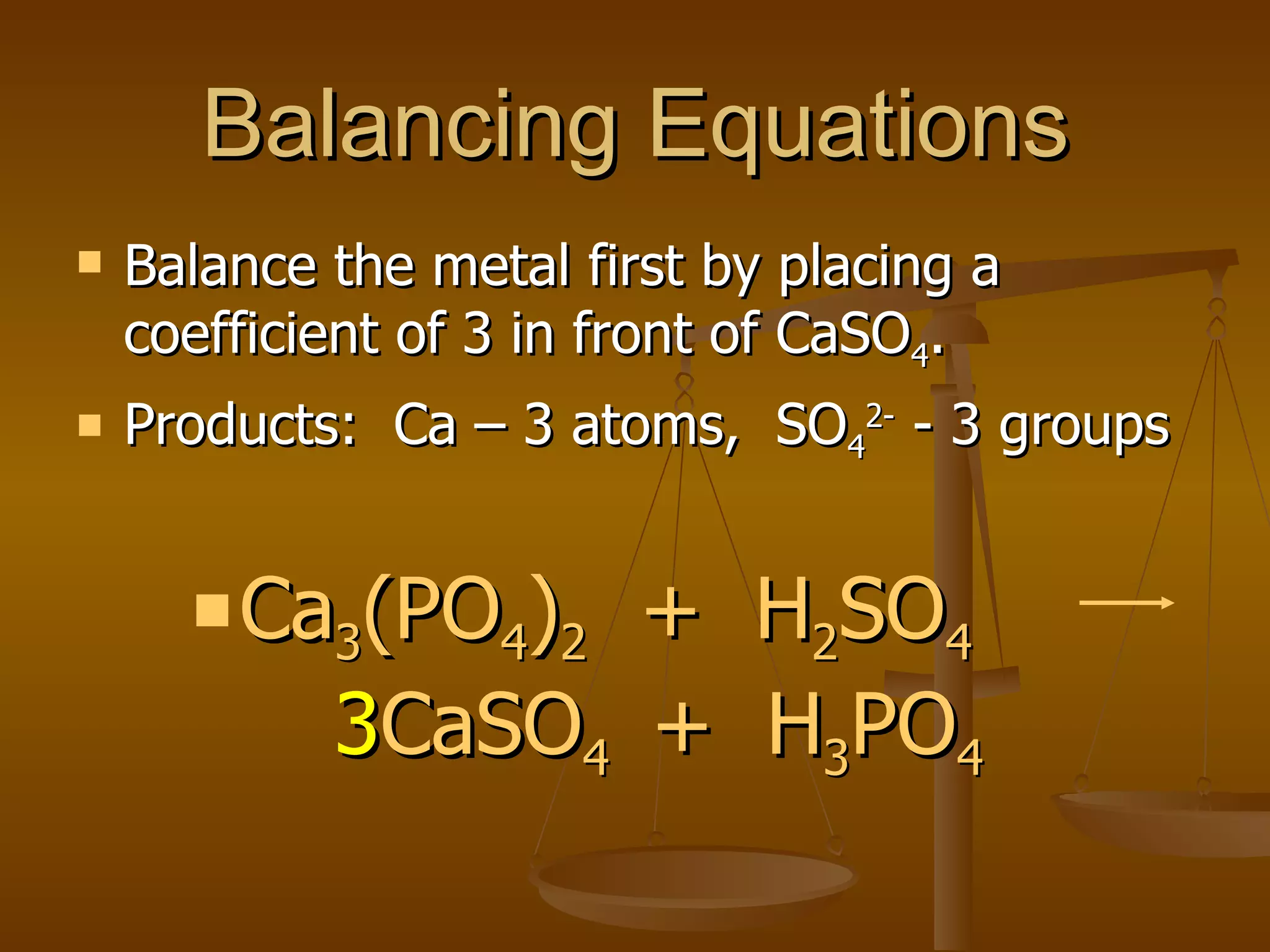
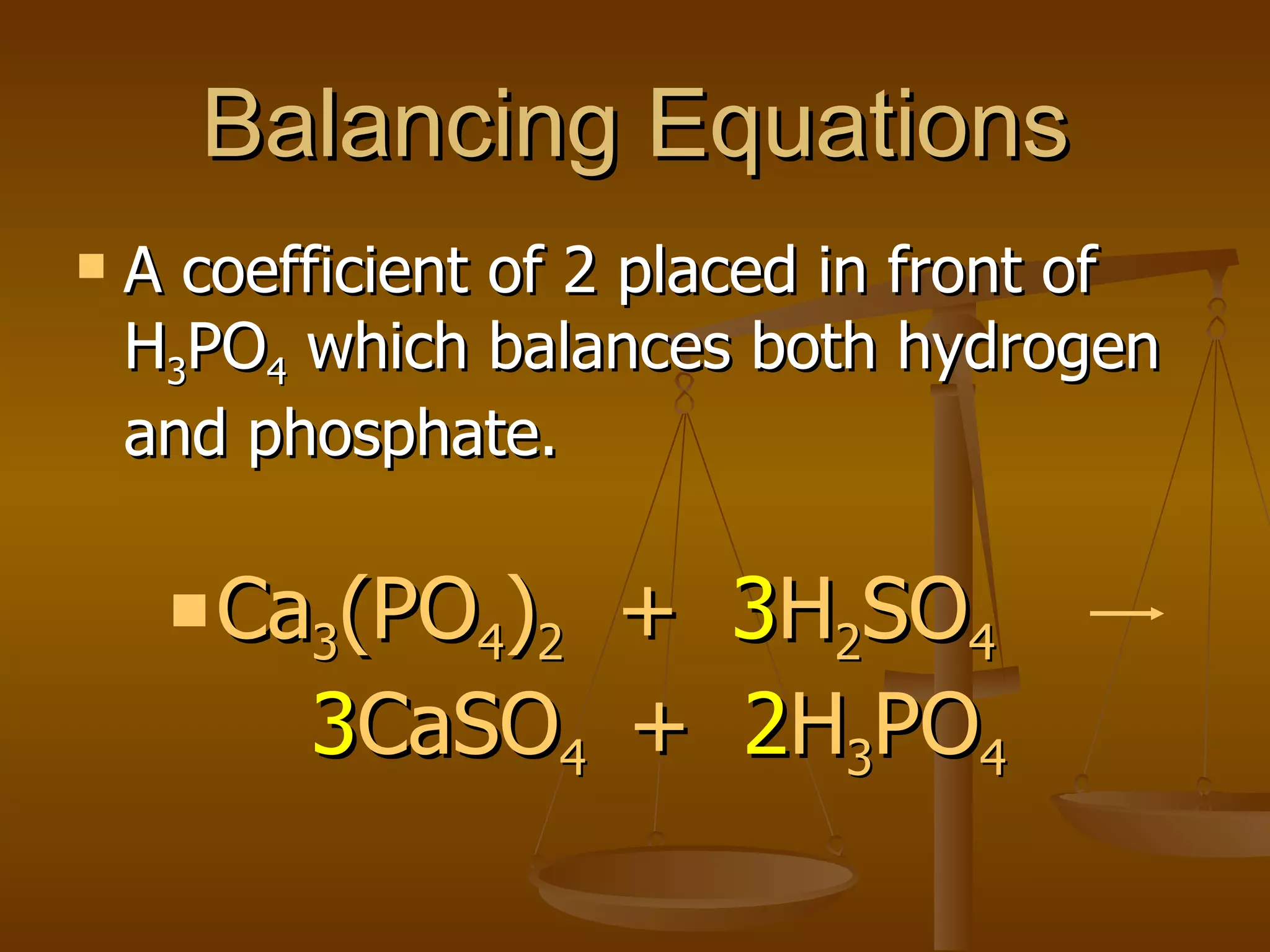
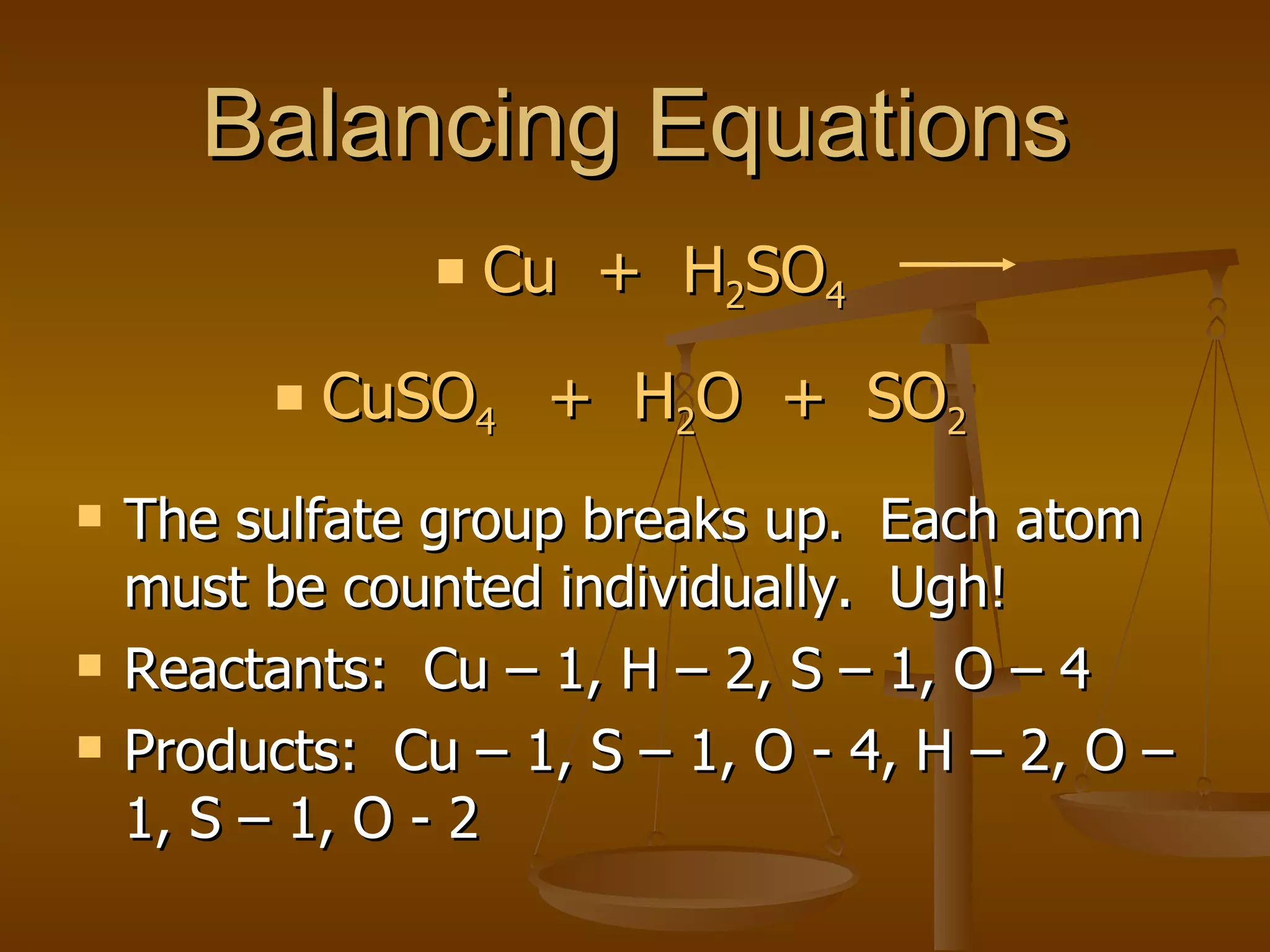
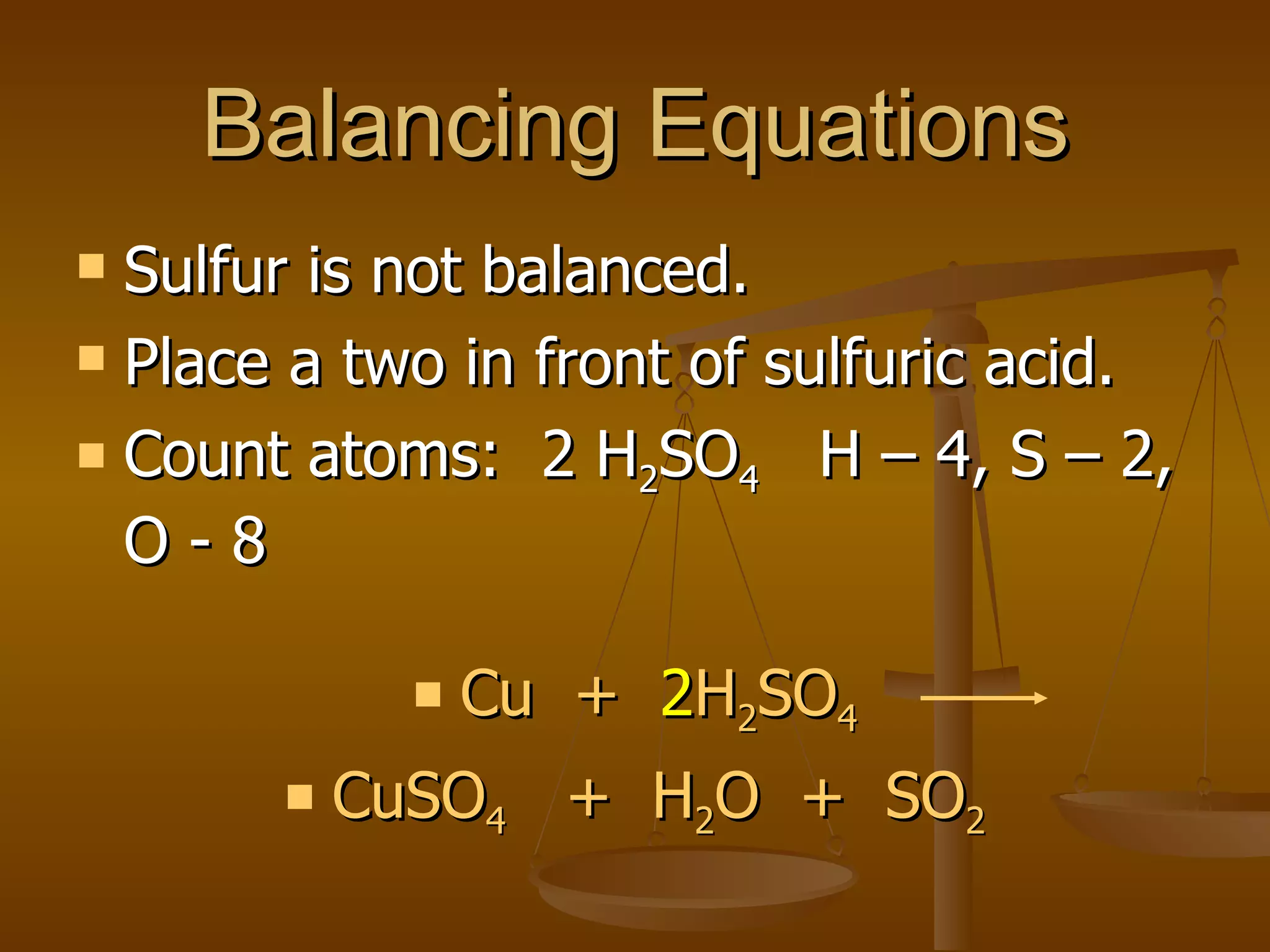
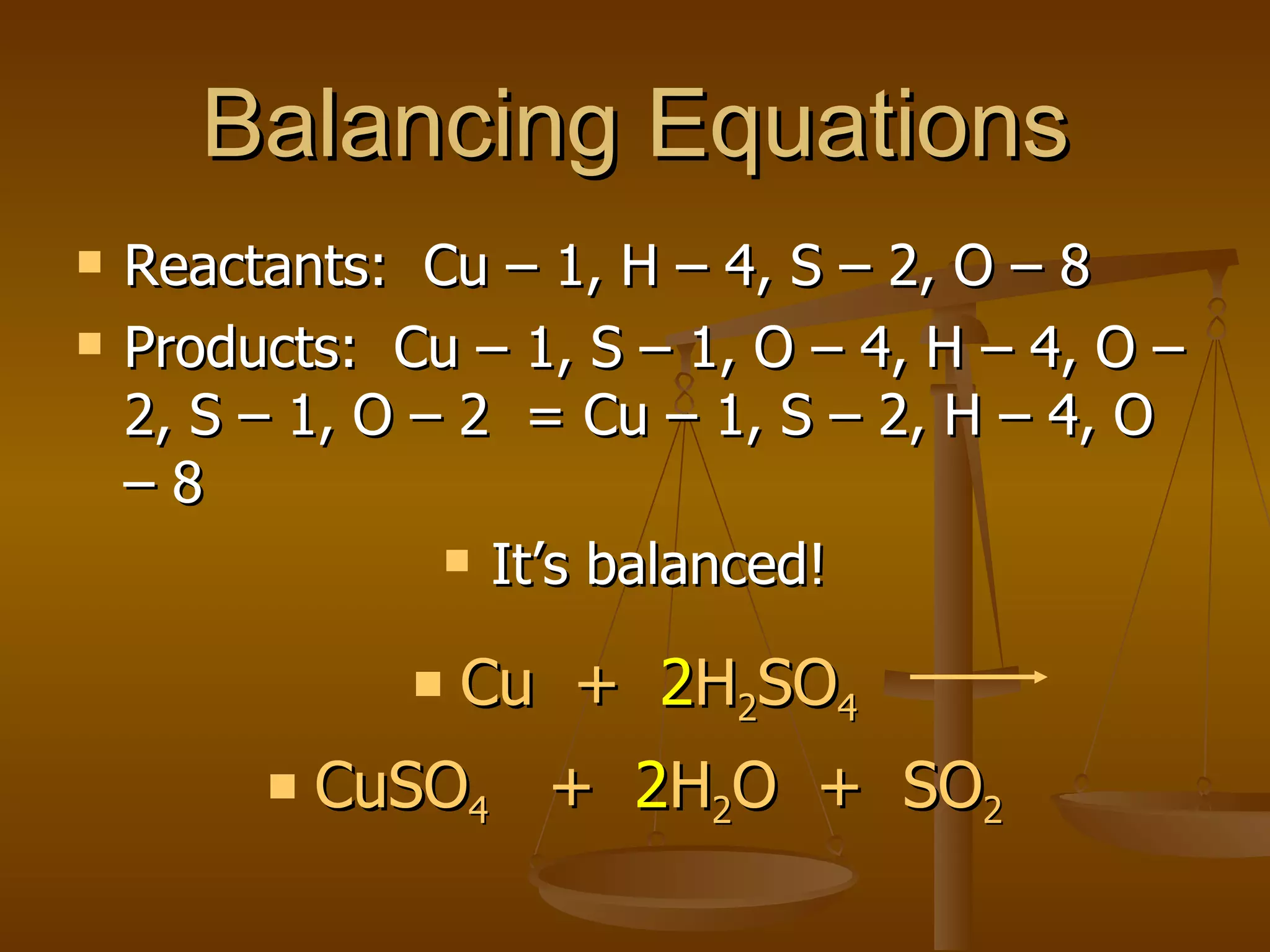
Balancing chemical equations involves ensuring that the number of atoms of each element is equal on both sides of the reaction arrow. This is done through adjusting coefficients in front of chemical formulas as needed. The document provides several examples of balancing different chemical equations step-by-step by counting atoms, and adjusting coefficients to make the number of each type of atom equal on both sides.