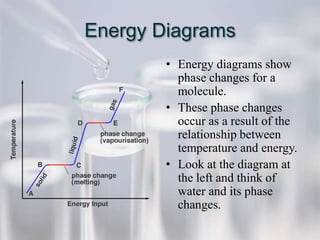
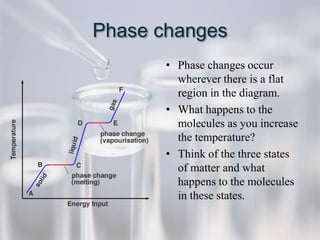
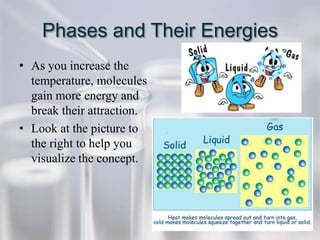
The document reviews key concepts for the third quarter assessment including the scientific method, bonding, naming compounds and ions, mixtures, the mole, energy diagrams, phases of matter, reaction rates, and gas laws. It provides examples and explanations of important terms and concepts to help students prepare.