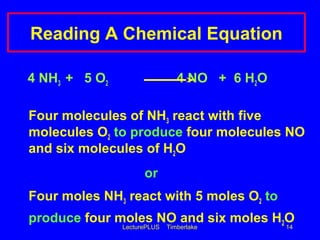
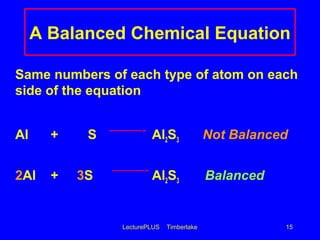
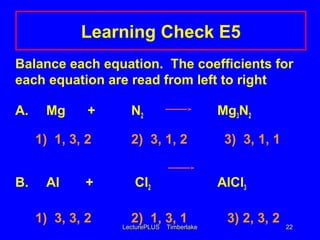
This document contains lecture slides about chemical and physical changes and chemical reactions. It discusses the differences between physical and chemical changes, how chemical equations are used to represent chemical reactions, and how to balance chemical equations. It provides examples of classifying changes as physical or chemical and examples of writing and balancing chemical equations. It emphasizes that chemical equations must satisfy the law of conservation of mass by having the same number and type of atoms on both sides.