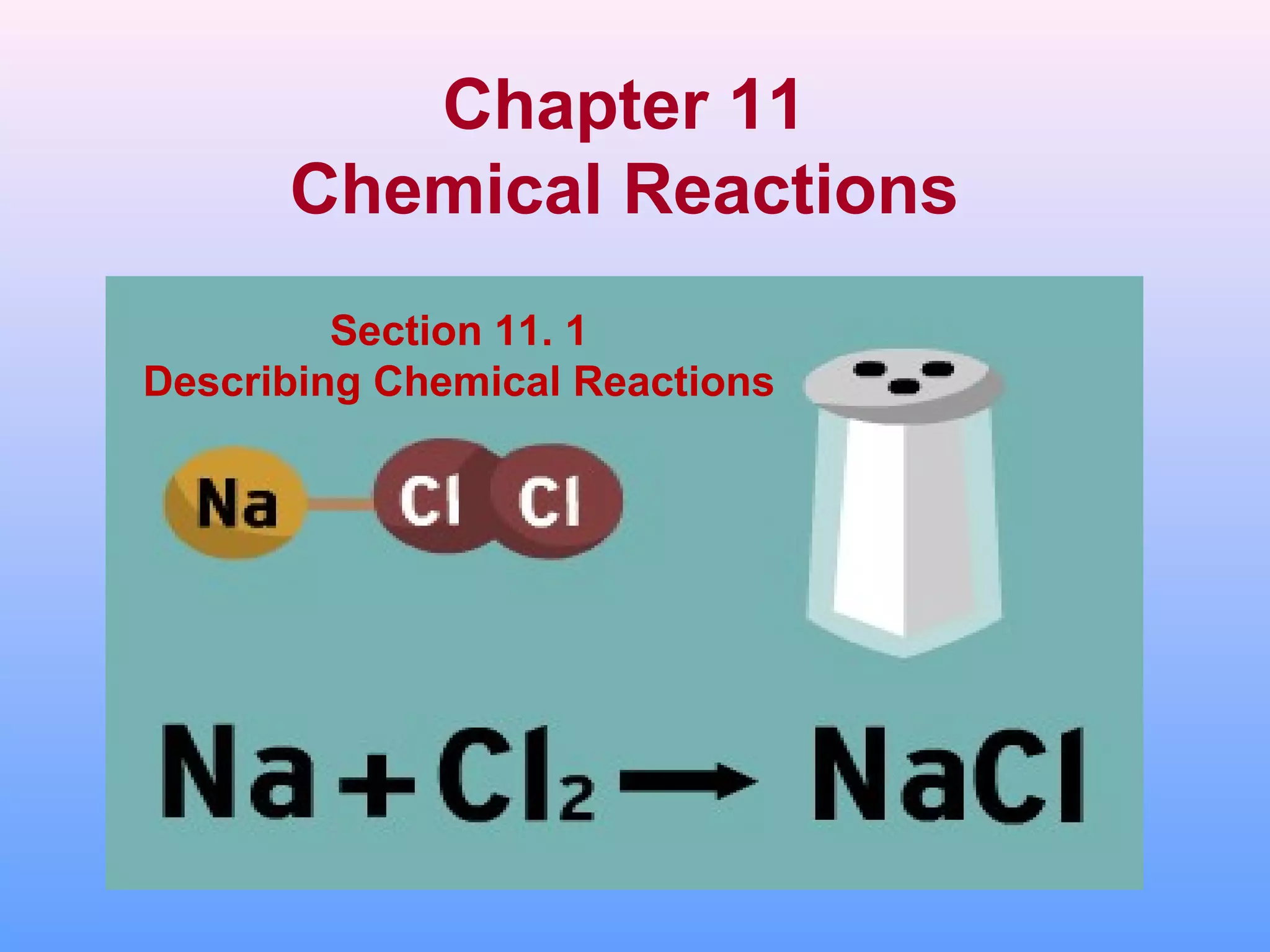
This document describes chemical reactions and equations. It discusses:
1. The components of chemical equations including reactants and products.
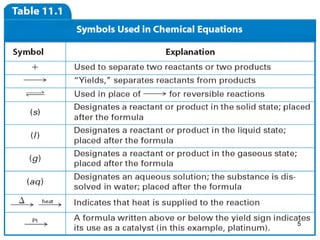
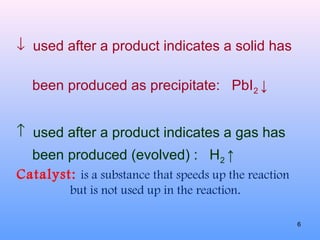
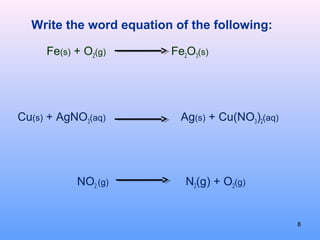
2. The different types of chemical equations including word equations, skeleton equations, and chemical equations.
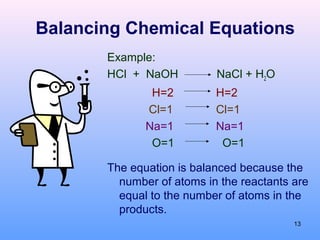
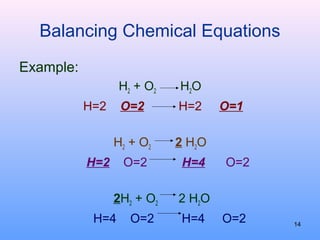
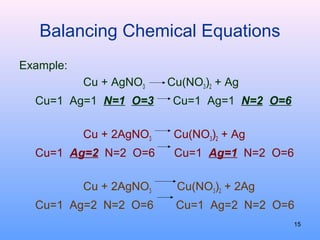
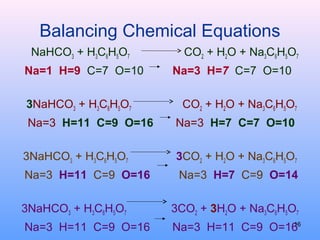
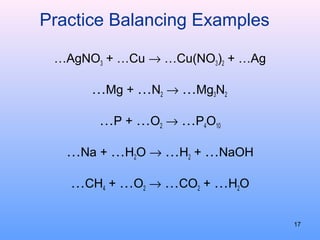
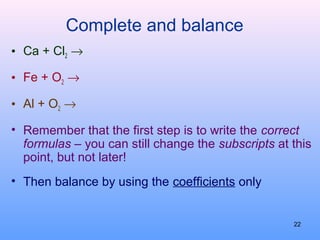
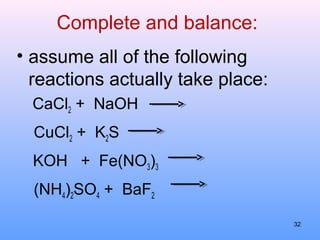
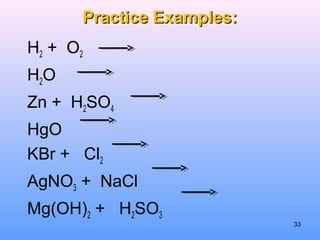
3. How to balance chemical equations by ensuring equal numbers of each type of atom on both sides of the reaction arrow.
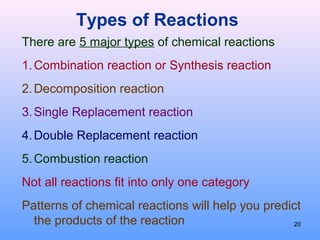
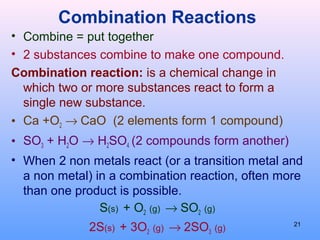
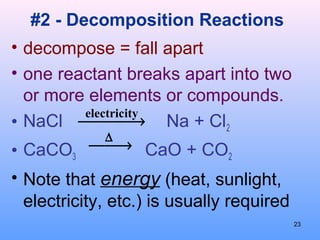
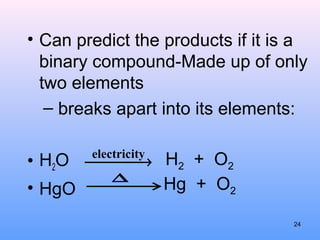
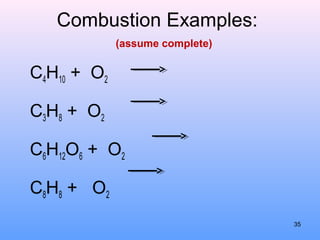
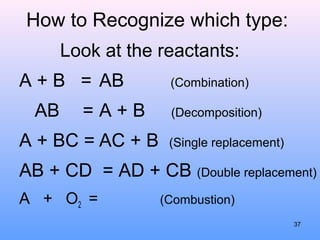
4. The five major types of chemical reactions - combination, decomposition, single replacement, double replacement, and combustion.
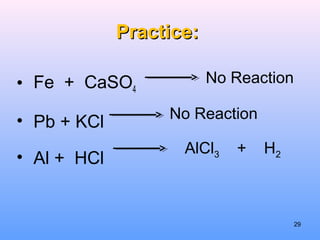
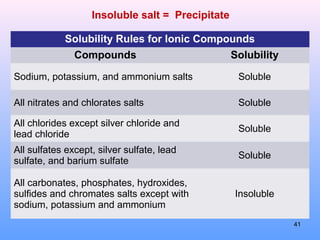
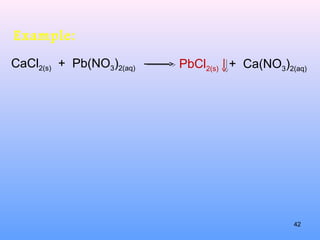
5. How to predict whether a precipitation reaction will occur based on solubility rules for ionic compounds.