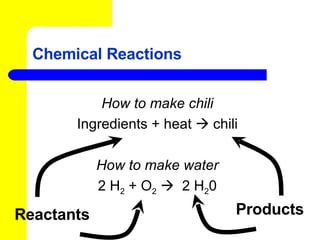
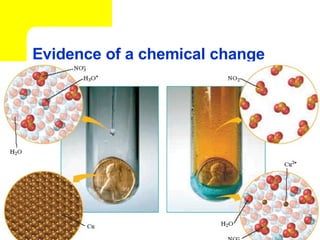
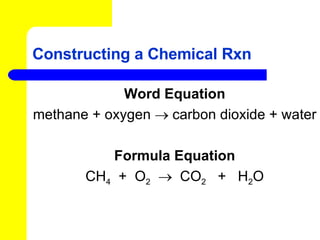
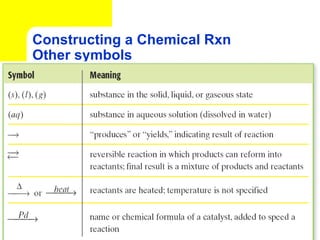
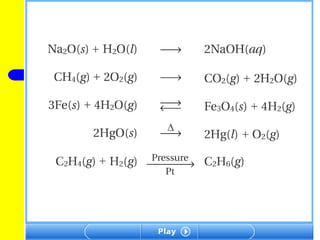
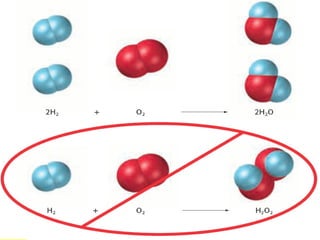
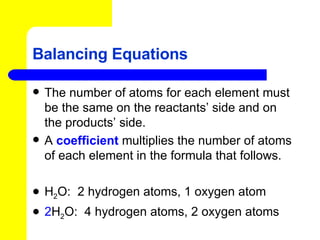
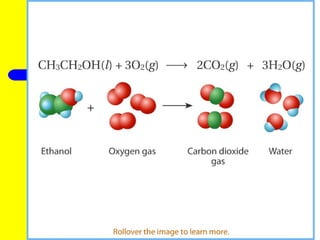
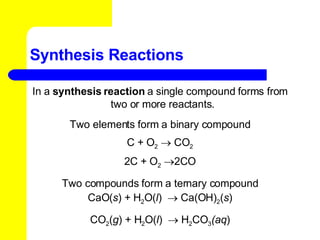
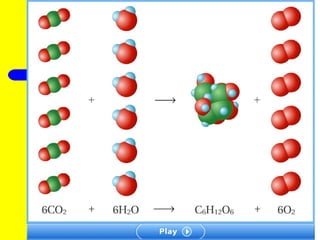
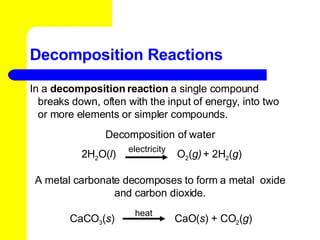
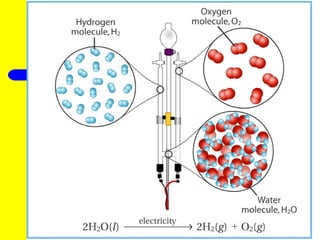
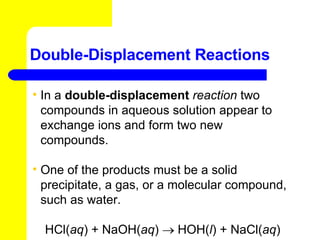
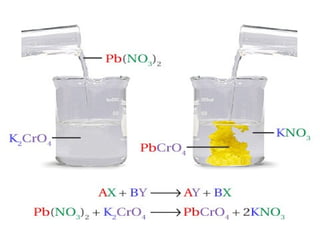
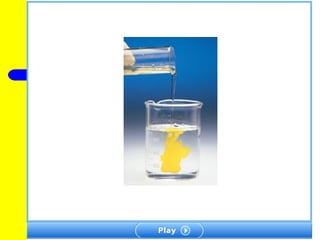
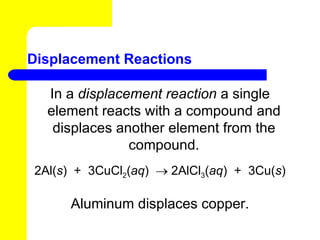
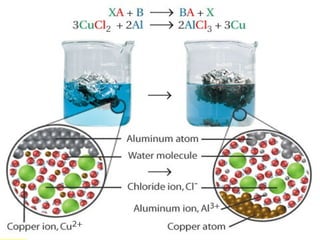
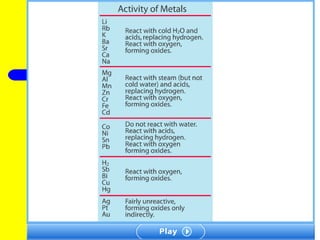
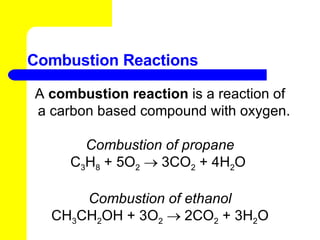
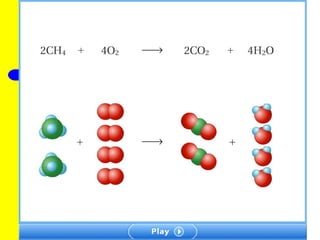
The document provides information about chemical reactions, including defining reactants and products, describing types of chemical reactions like synthesis, decomposition, double displacement, displacement and combustion reactions. It also discusses how to write and balance chemical equations.