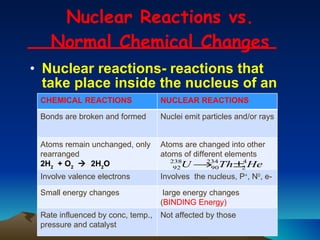
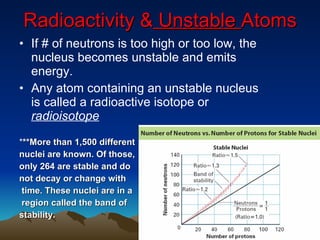
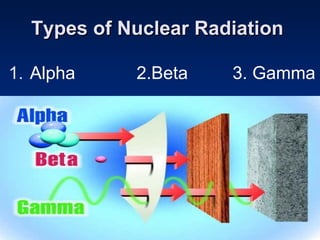
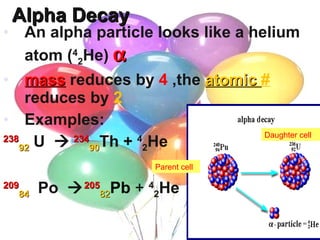
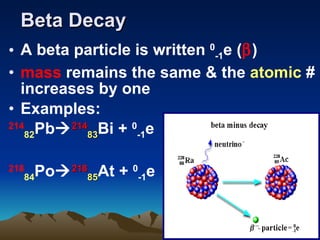
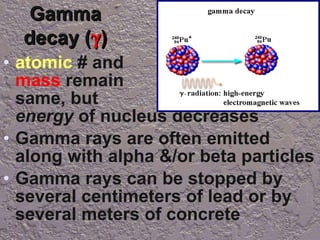
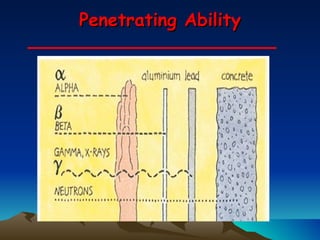
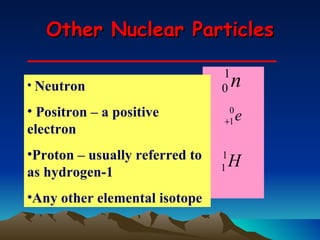
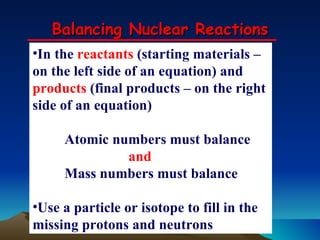
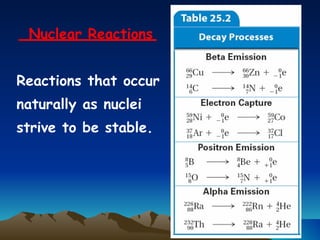
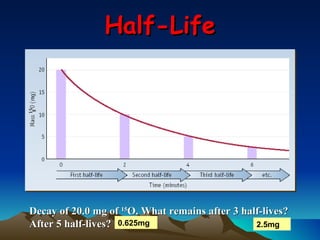
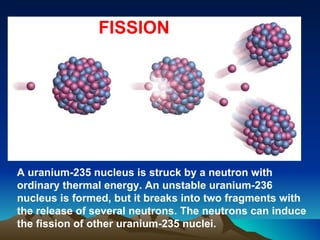
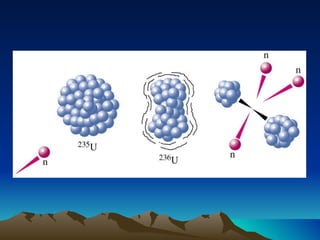
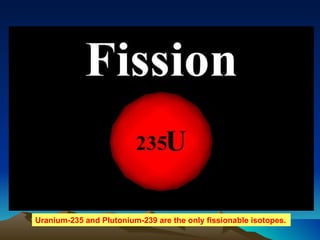
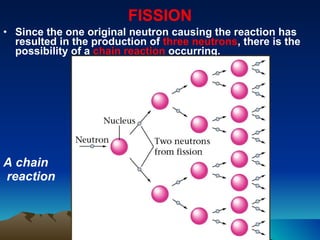
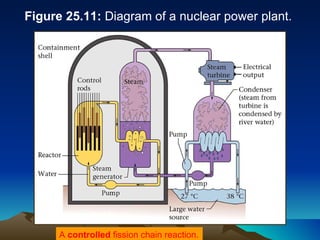
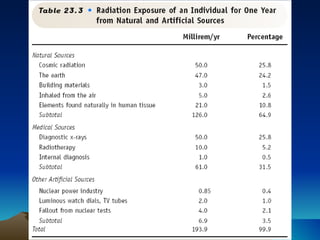
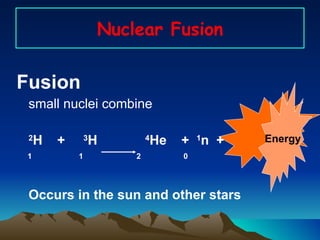
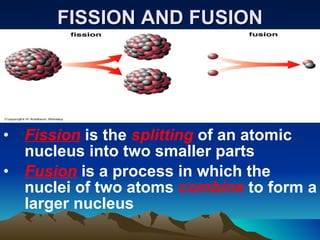
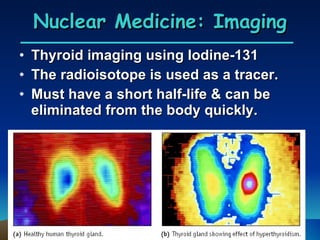
Nuclear reactions involve changes to atomic nuclei, whereas chemical reactions only involve rearrangement of electrons. Nuclear reactions can release large amounts of energy and produce new elements, while chemical reactions typically involve smaller energy changes and atoms remain the same elements. Radioactive decay is an example of a nuclear reaction where an unstable atomic nucleus spontaneously transforms into a more stable nucleus by emitting particles or electromagnetic radiation.