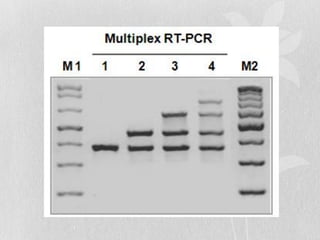
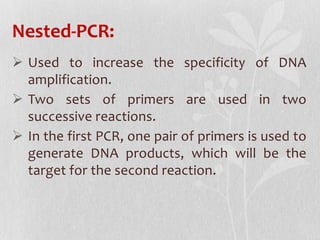
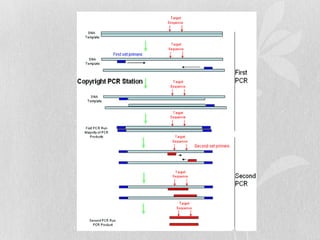
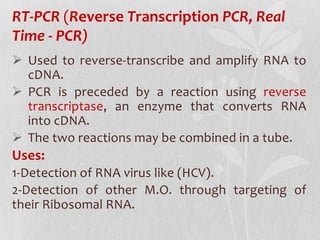
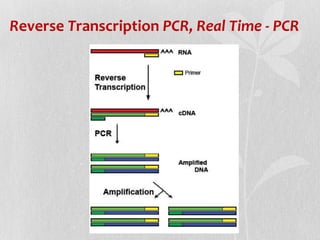
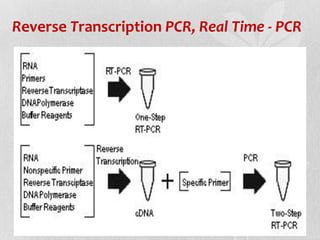
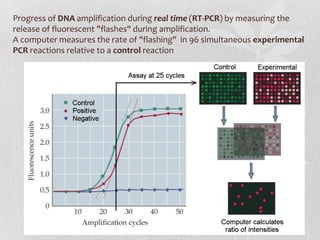
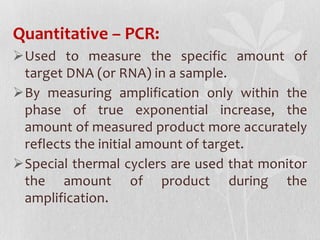
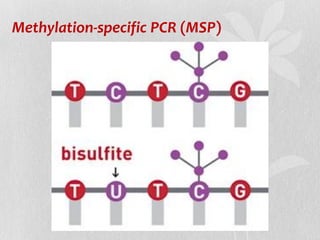
This document discusses different types of polymerase chain reaction (PCR) techniques. It begins by providing background on PCR and its development. It then describes several types of PCR including multiplex PCR, which allows for simultaneous detection of multiple pathogens; nested PCR, which increases specificity; reverse transcription PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR), which are used to detect RNA; quantitative PCR, which measures specific target DNA/RNA amounts; and other variants like hot-start PCR, touchdown PCR, and methylation-specific PCR. Each type is briefly explained along with its uses and applications in medical research.