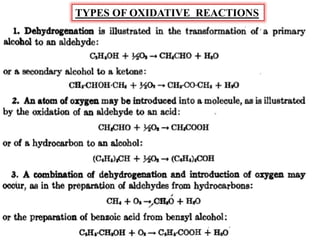
The document discusses various oxidative reactions and the role of non-metallic oxidizing agents like hydrogen peroxide (H2O2) and sodium hypochlorite (NaOCl). It details the production methods of hydrogen peroxide, its powerful oxidizing properties, and its industrial applications, including its ability to dechlorinate effluent and oxidize organic compounds. Additionally, it covers the production of sodium hypochlorite and its uses in cleaning, disinfection, and wastewater treatment, as well as an overview of ozonolysis and its implications in organic chemistry and industrial processes.