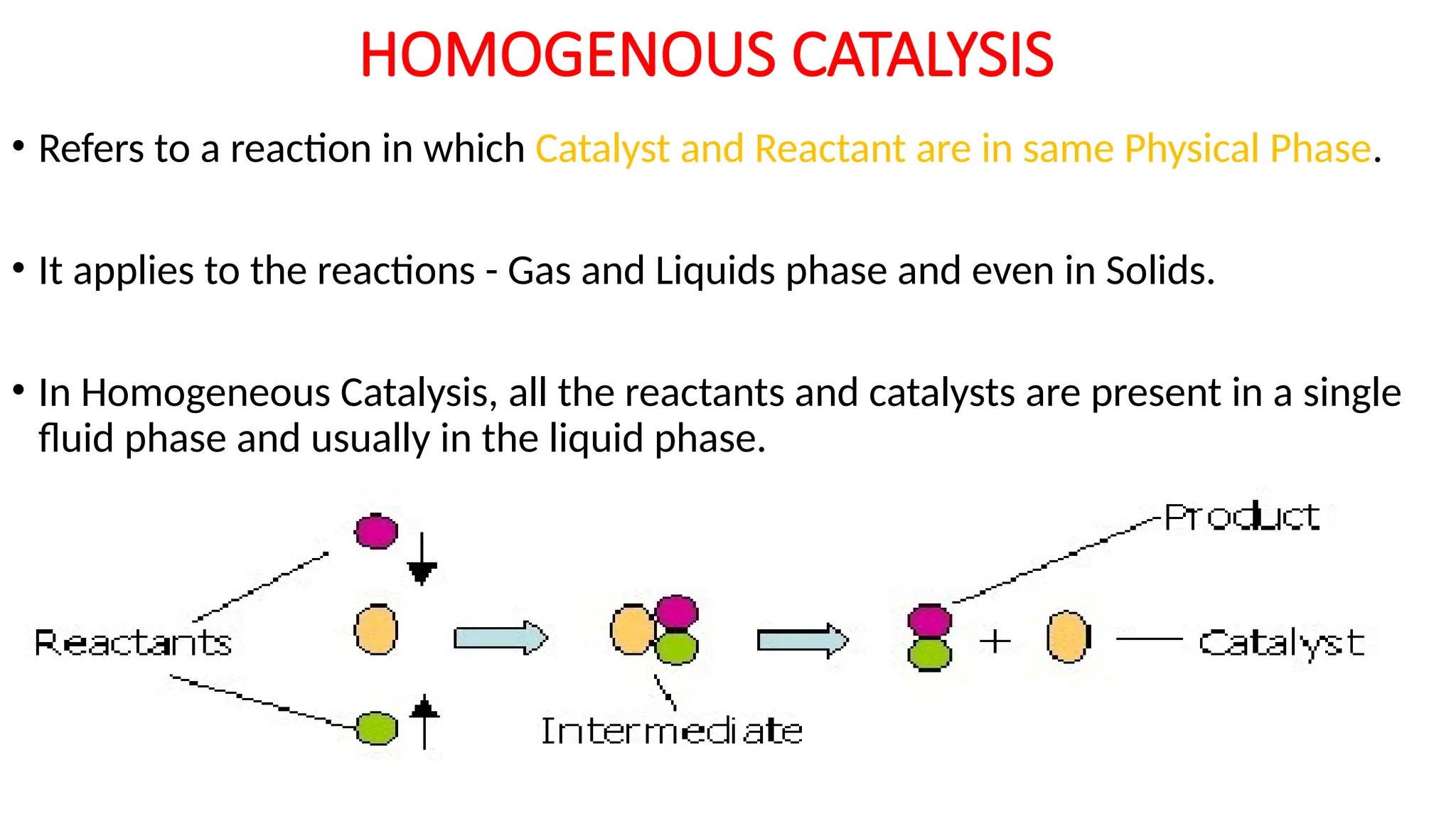
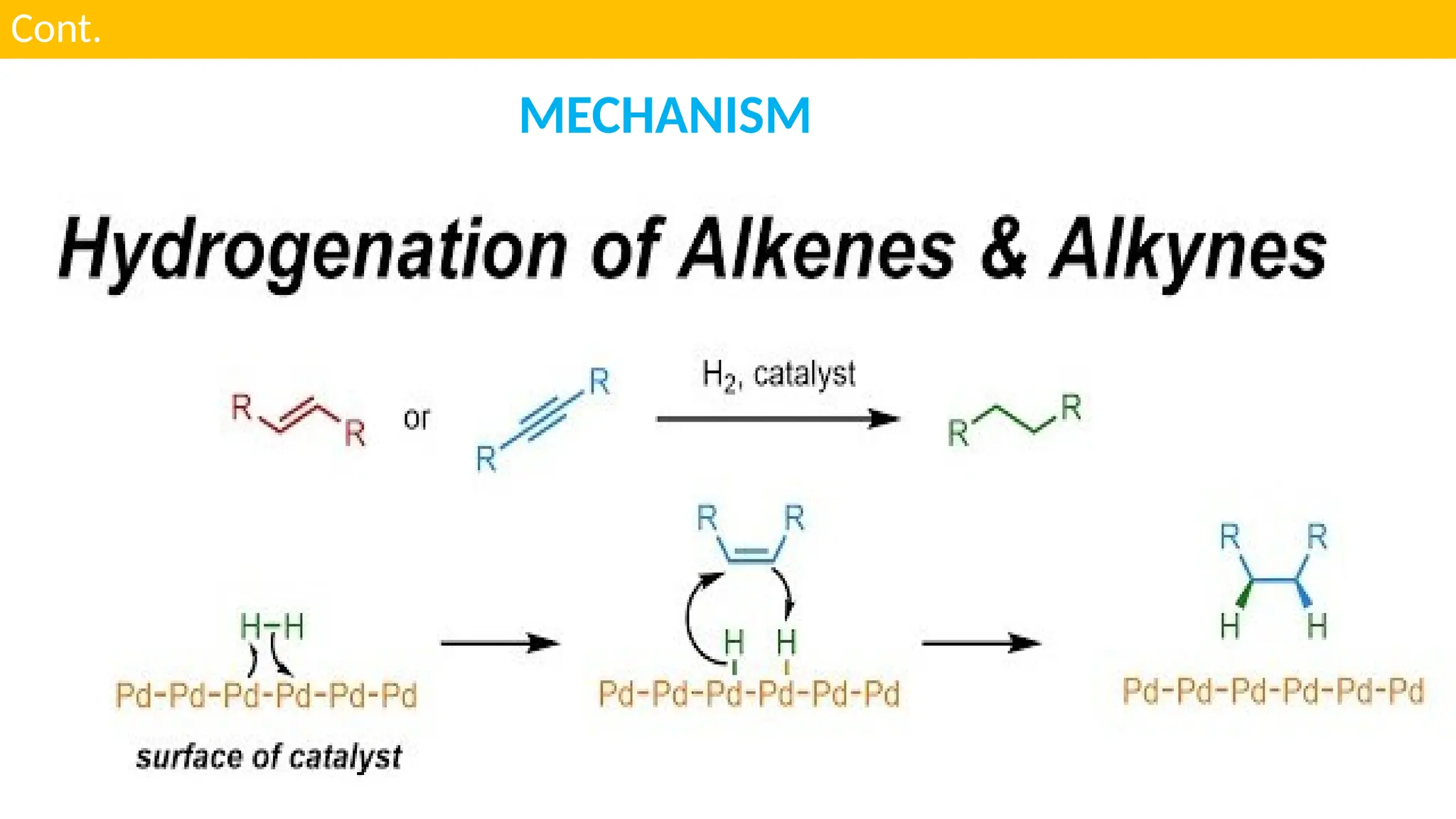
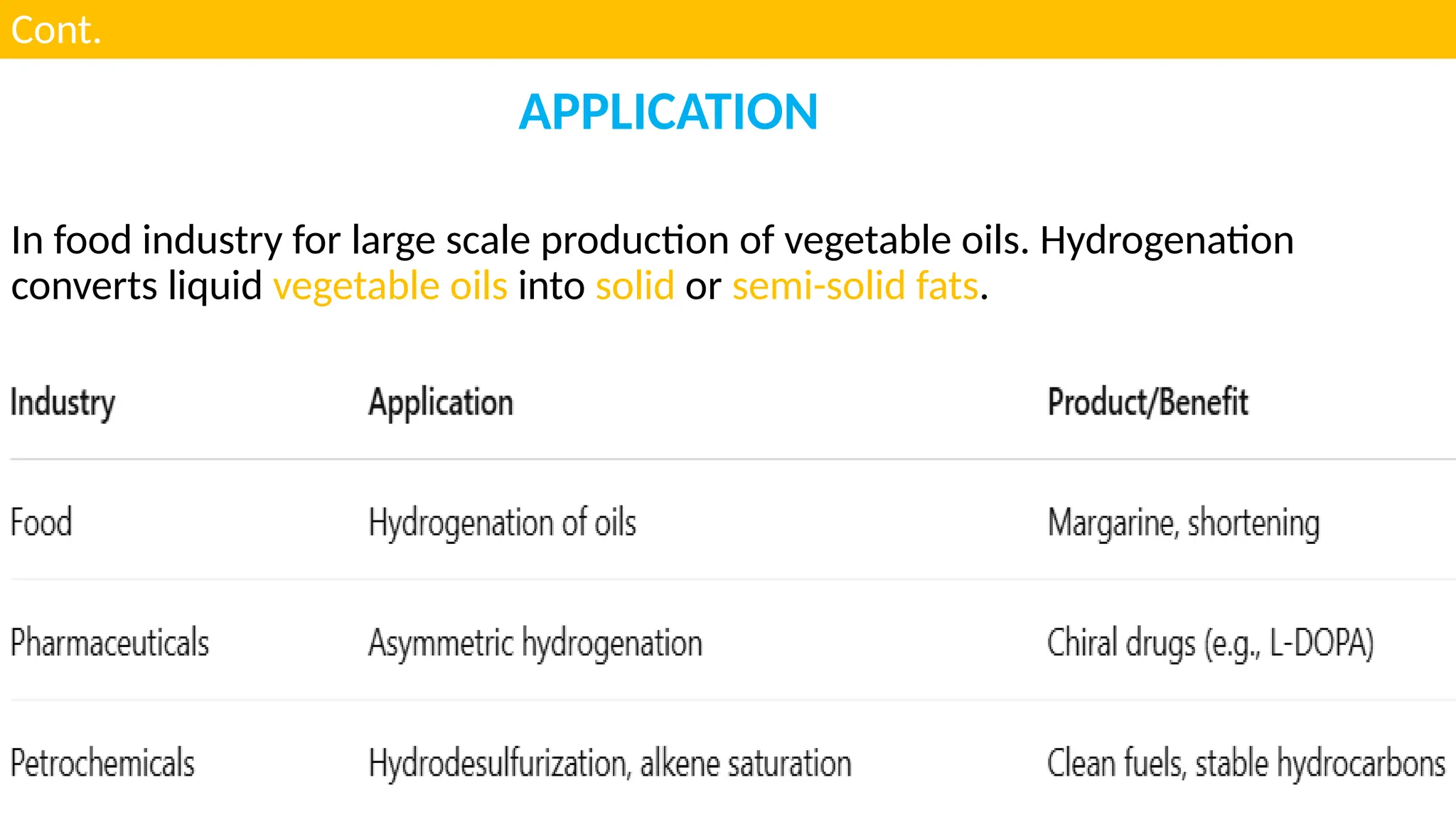
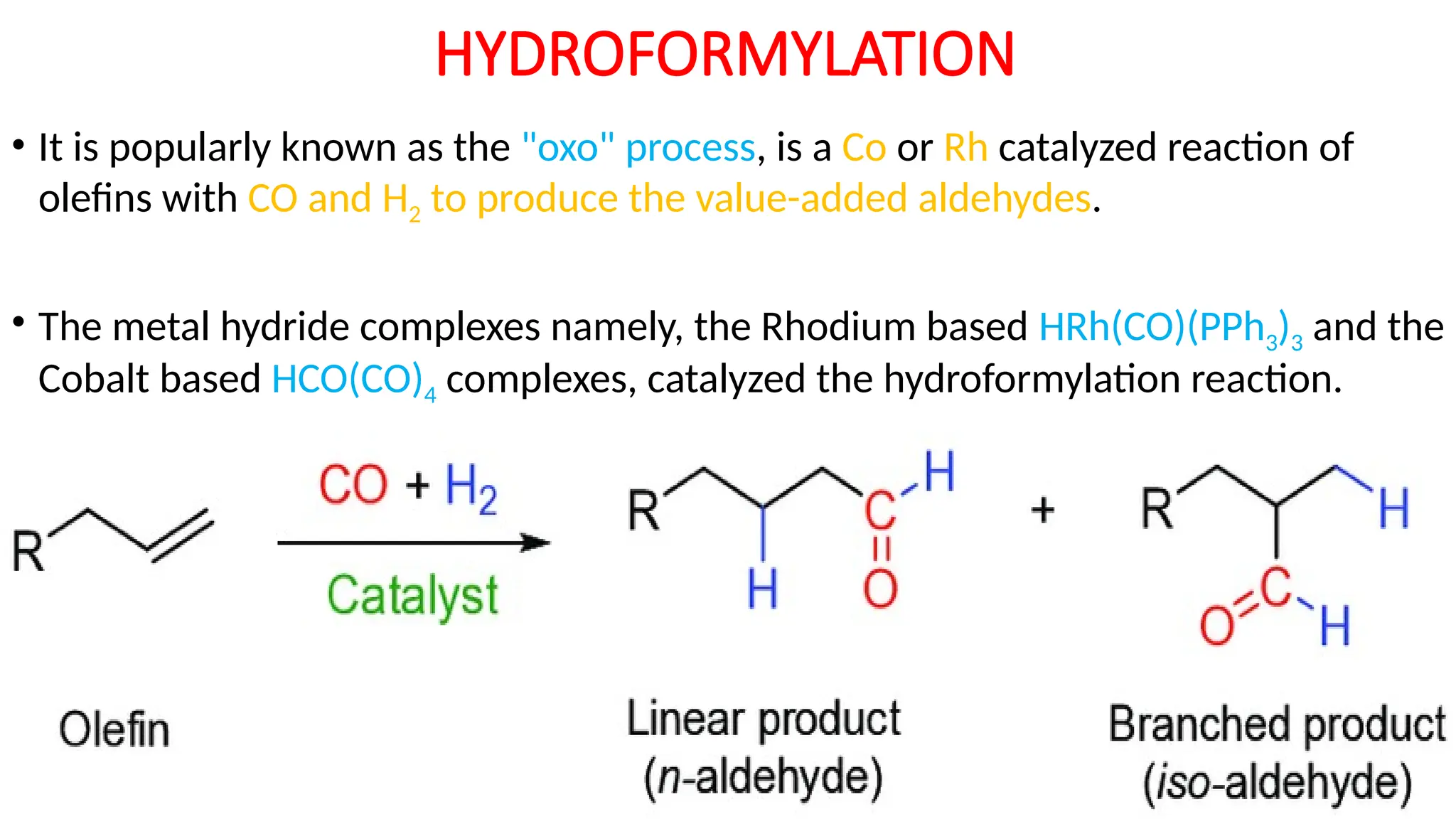
M.PHARMA CHEMISTRY 2ND SEM
(MPC202T) ADVANCED ORGANIC CHEMISTRY - 2
MECHANISMS AND APPLICATIONS
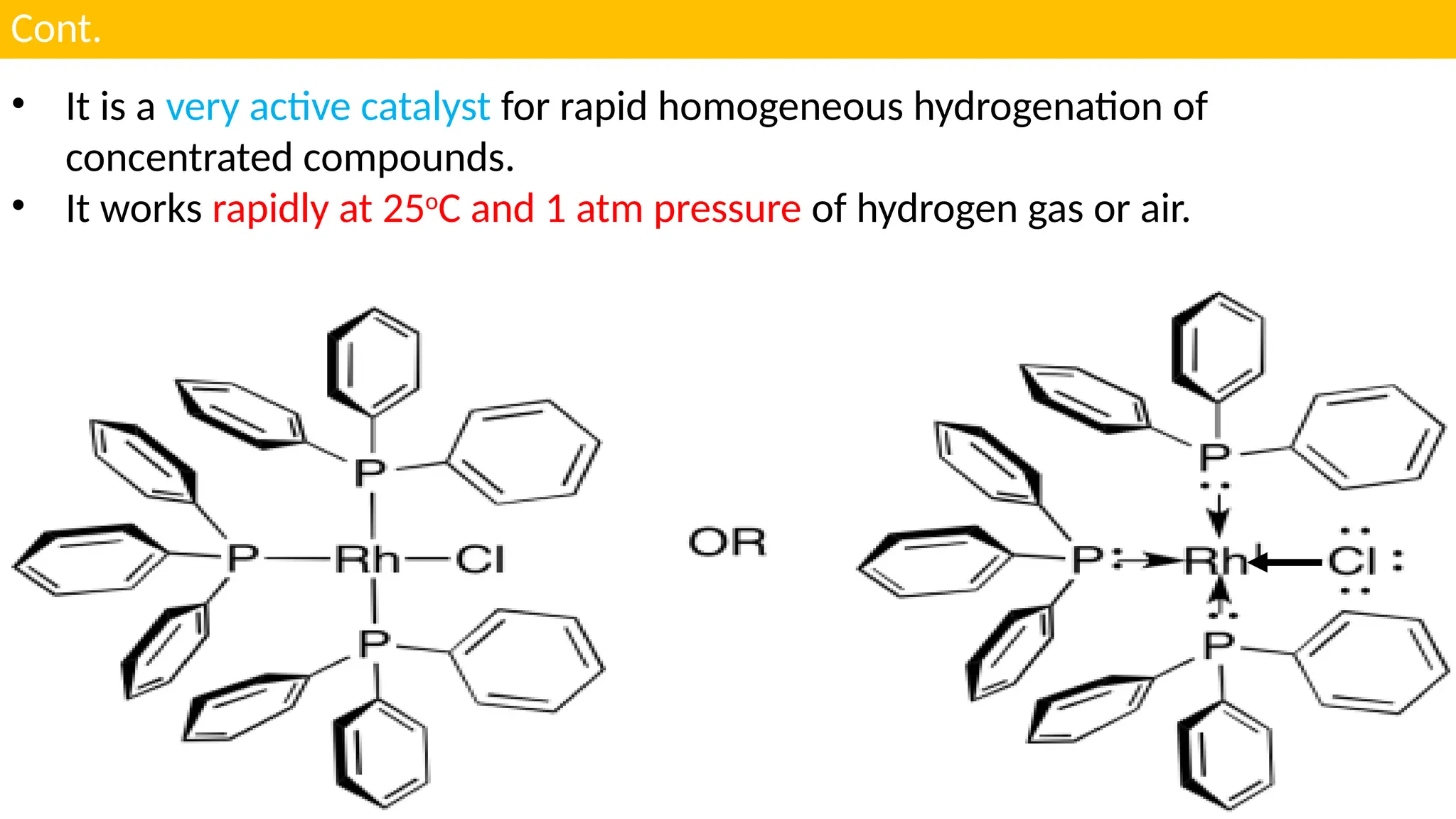
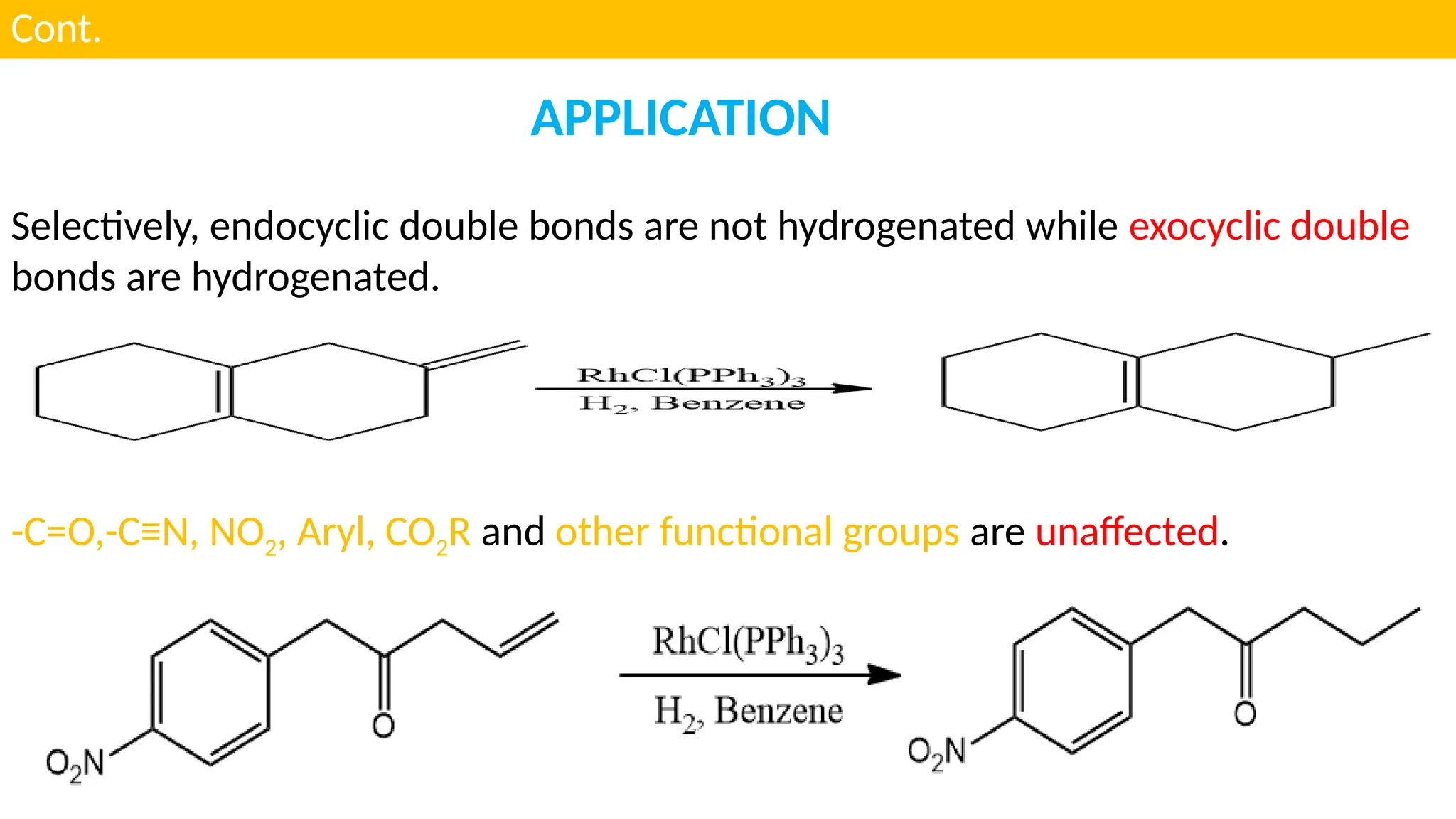
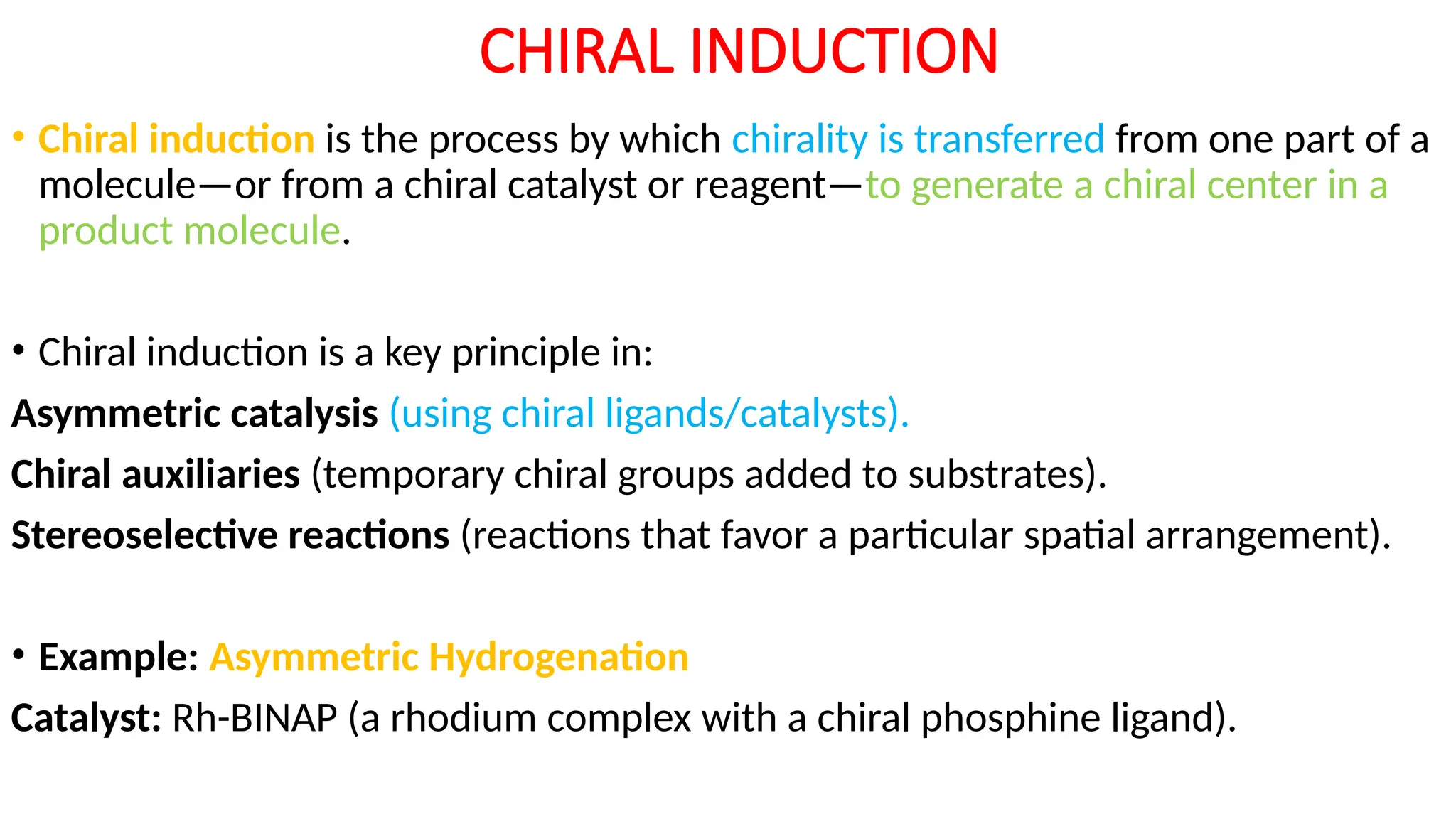
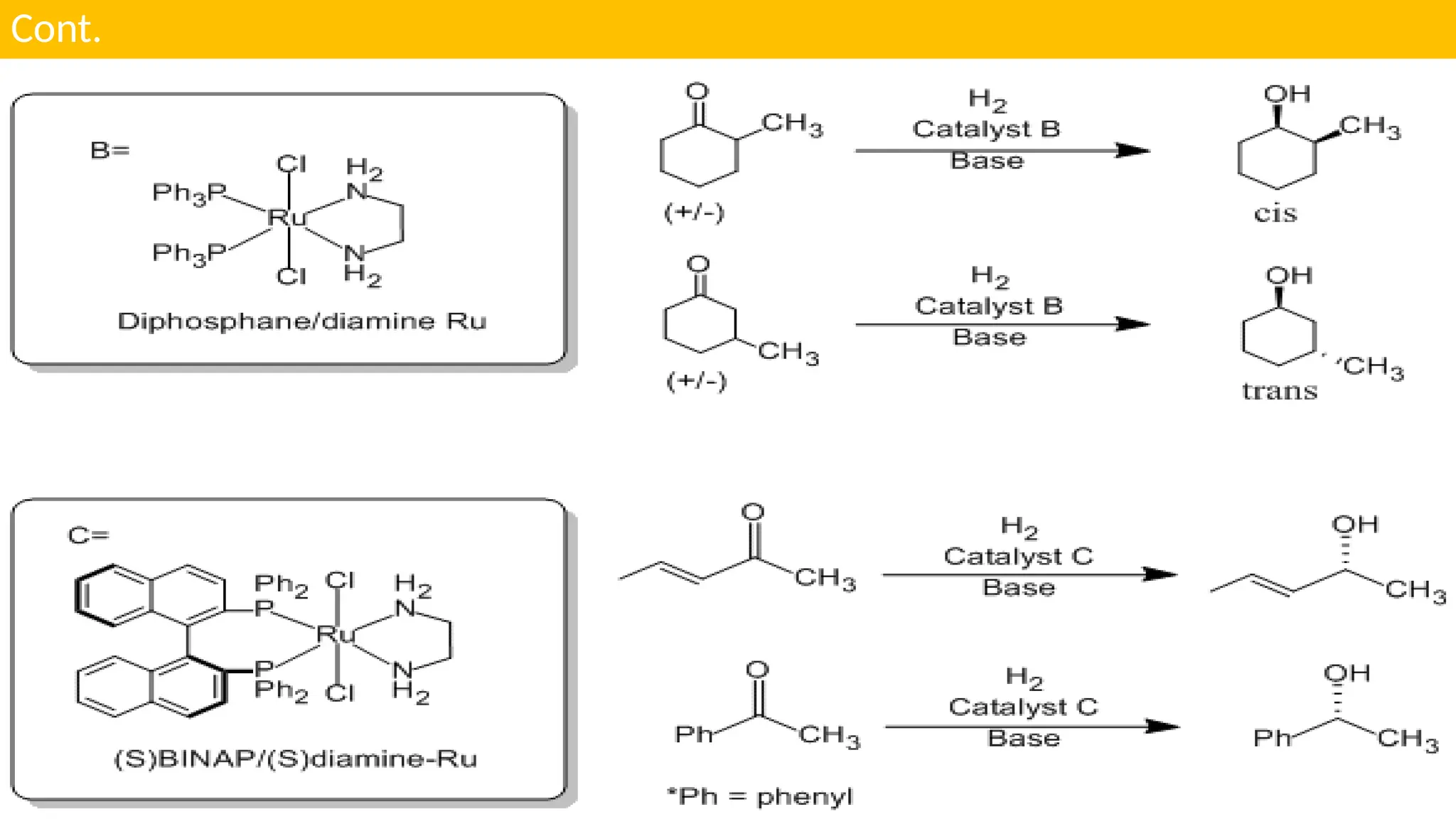
UNIT - 4 CATALYSIS : (C) HOMOGENOUS CATALYSIS , HYDROGENATION , HYDROFORMYLATION , HYDROCYANATION , WILKINSON CATALYSTS , CHIRAL LIGANDS & CHIRAL INDUCTION , ZIEGLER-NATTA CATALYSTS , SOME EXAMPLES OF HOMOGENOUS CATALYSIS USED IN SYNTHESIS OF DRUGS