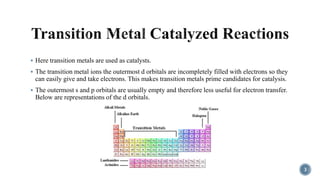
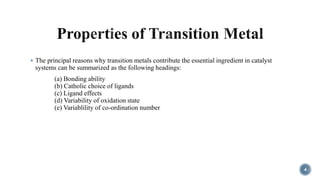
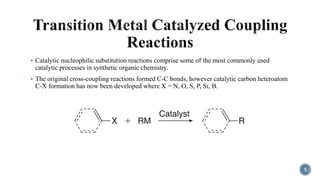
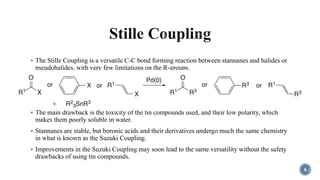
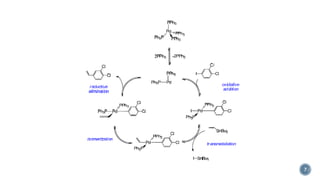
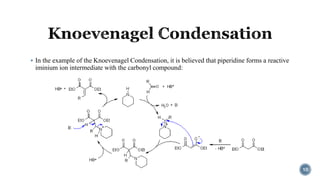
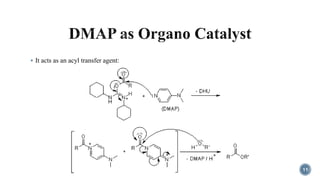
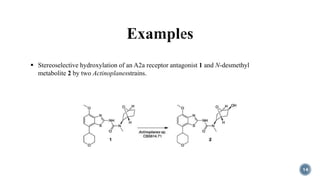
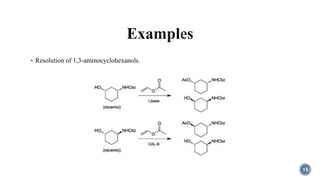
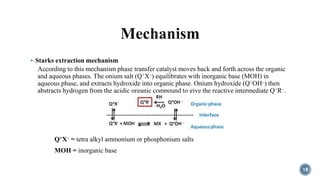
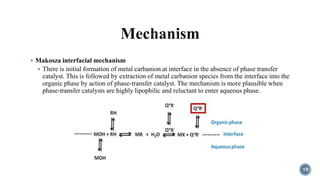
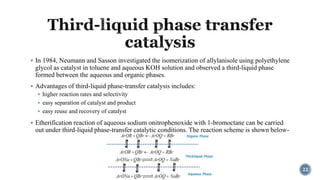
The document discusses various catalytic methods in organic synthesis, emphasizing transition metal catalysis, organo-catalysis, and biocatalysis. It highlights the significance of transition metals due to their unique electron transfer capabilities and examines popular reactions like the Stille and Suzuki couplings involving organoboron reagents. Additionally, it outlines phase-transfer catalysis as a means to facilitate reactant migration between phases, enhancing reaction efficiency.









![ Meyer H, Eichhorn E, Hanlon S, Lütz S, Schürmann M, Wohlgemuth R et al. The use of
enzymes in organic synthesis and the life sciences: perspectives from the Swiss Industrial
Biocatalysis Consortium (SIBC). Catal Sci Technol. 2013;3(1):29-40.
Clayden J, Greeves N, Warren S. Organic Chemistry. 2nd ed. New Delhi: Oxford University
Press; 2001.
Phase Transfer Catalysis [Internet]. NPTEL. 2014 [cited 30 July 2014]. Available from:
http://nptel.ac.in/courses/103103026/44
24](https://image.slidesharecdn.com/catalysis-180419055602/85/Catalysis-24-320.jpg)
