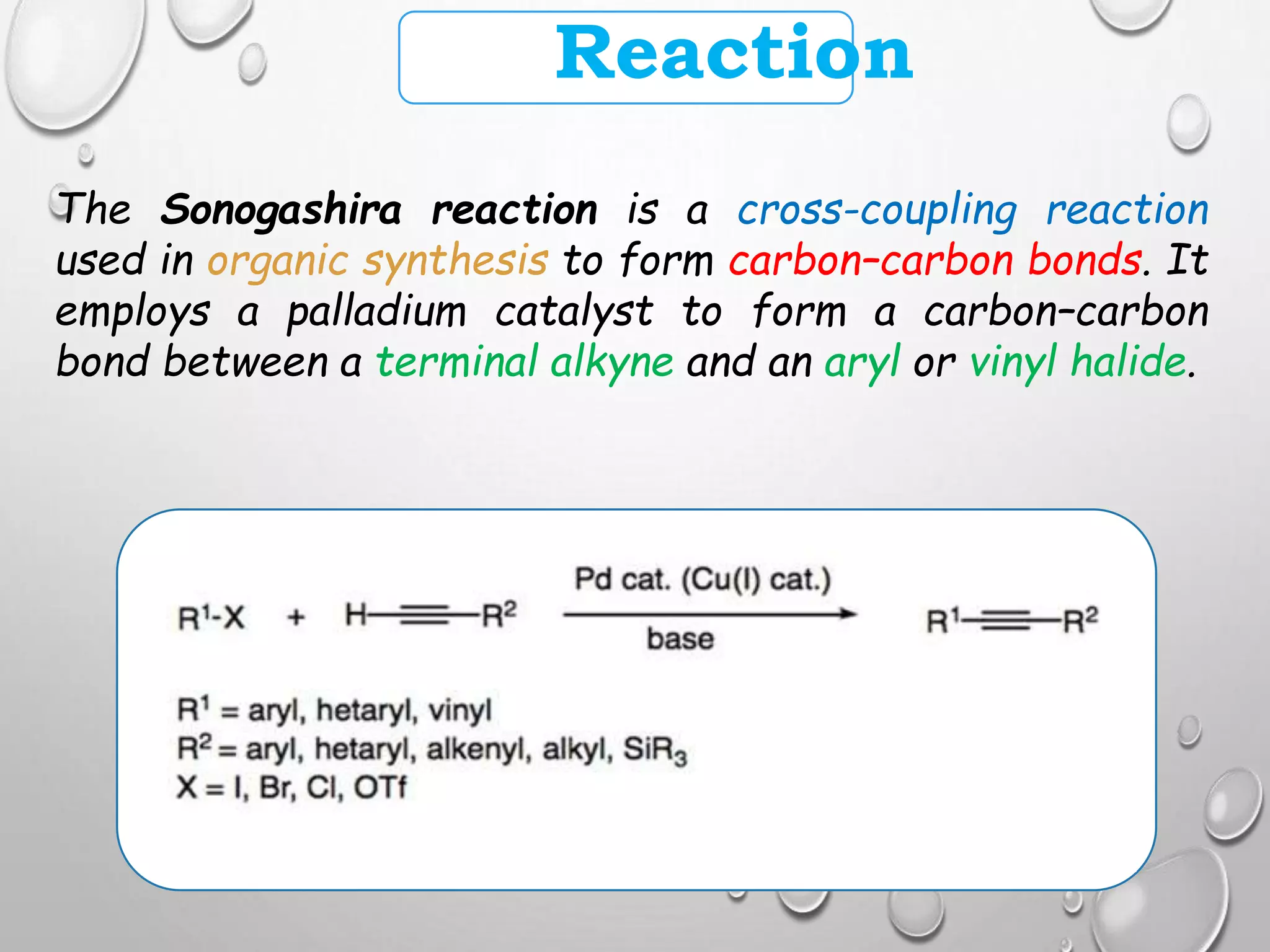
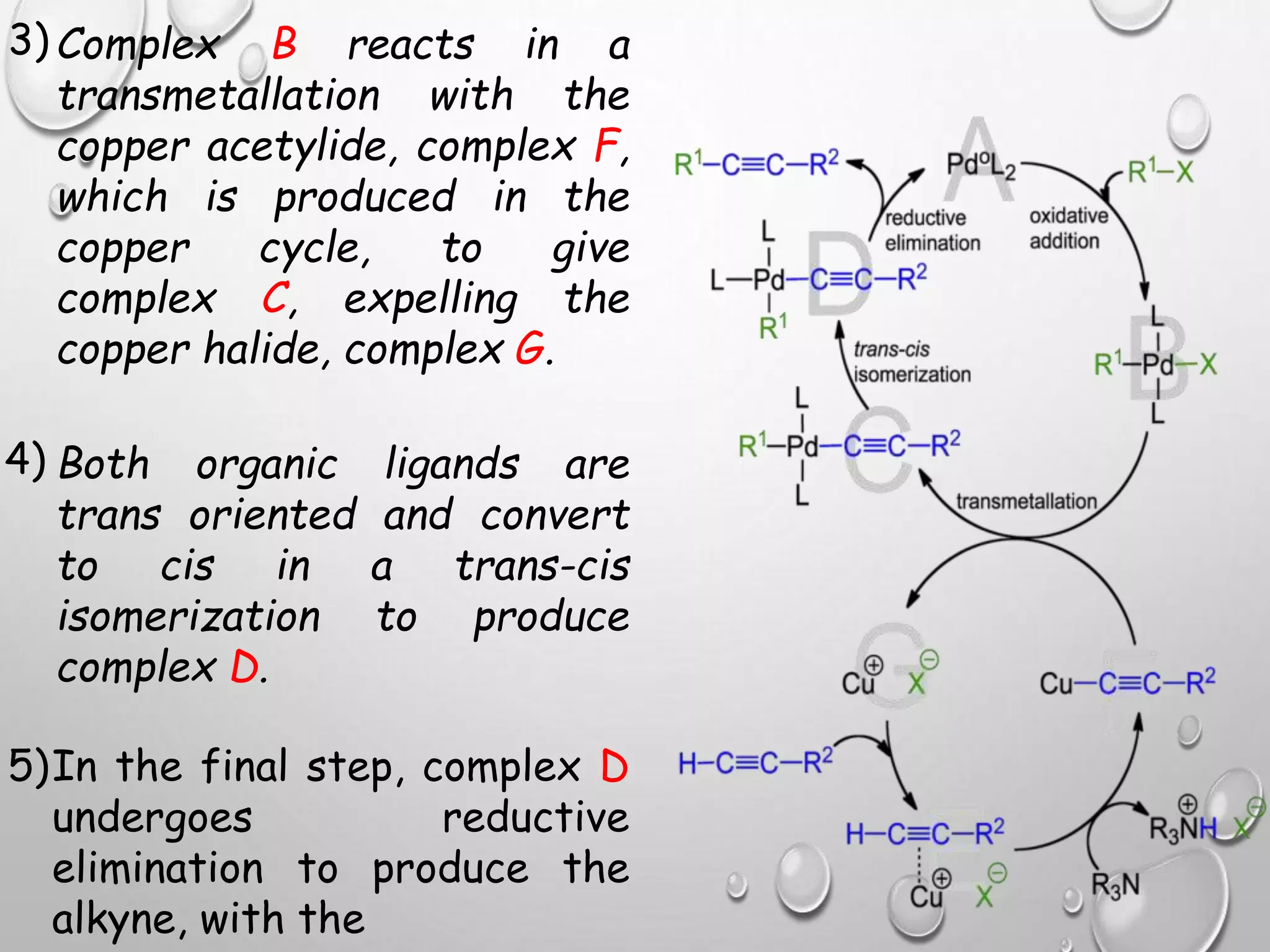
The Sonogashira cross-coupling reaction forms carbon-carbon bonds between a terminal alkyne and an aryl or vinyl halide using a palladium catalyst. It was developed in 1975 and offers milder conditions than previous coupling reactions, such as room temperature. The reaction employs both a palladium and copper catalyst, with the copper activating the alkyne. It has become a highly useful reaction for carbon-carbon bond formation and has applications in pharmaceuticals, natural products, and organic materials synthesis.