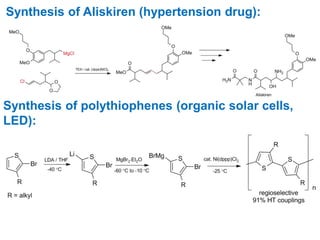
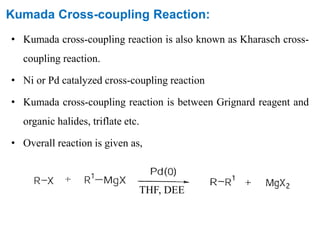
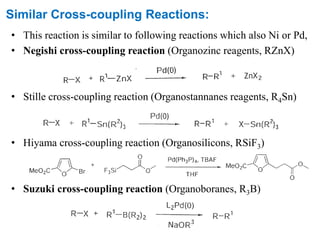
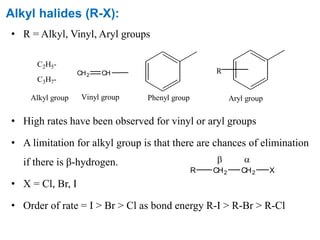
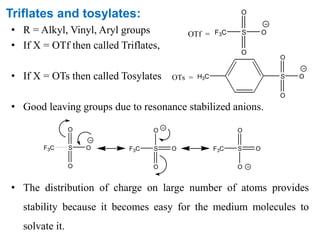
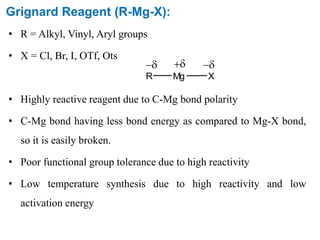
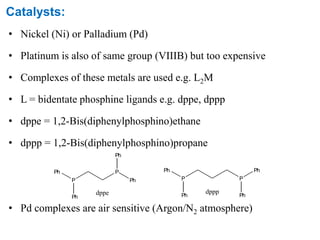
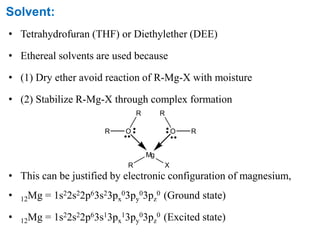
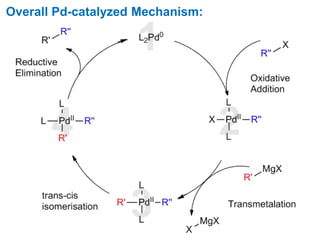
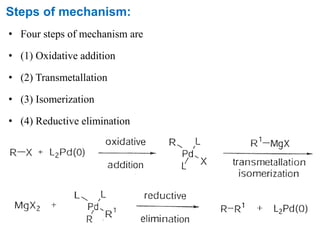
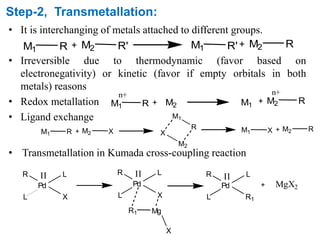
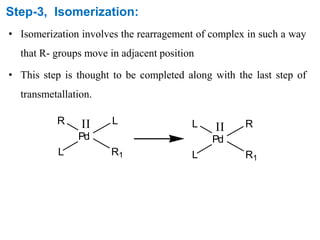
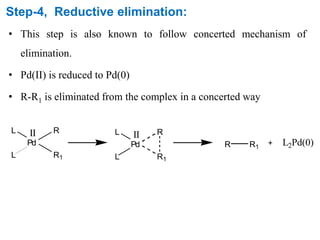
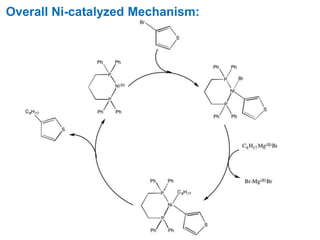
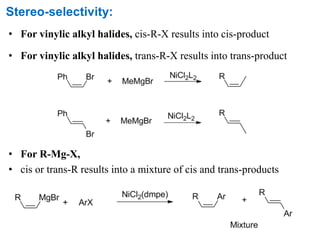
The Kumada cross-coupling reaction is a nickel or palladium catalyzed reaction between a Grignard reagent and an organic halide. The reaction proceeds through four steps - oxidative addition, transmetallation, isomerization, and reductive elimination. It is similar to other cross-coupling reactions like Negishi, Stille, Hiyama, and Suzuki reactions. The reaction exhibits cis/trans selectivity and can be made enantioselective using a chiral palladium catalyst. It has applications in synthesizing drugs like Aliskiren and materials for organic solar cells.



![Enantio-selectivity:
• Asymmetric synthesis by Pladium catalyst with chiral ligands
results into one enantiomeric product (>90%)
A: [Methoxyalkyl(ferrocenyl)] monophosphine
B: bis-oxazoline](https://image.slidesharecdn.com/kumadacross-couplingreaction-200717163613/85/Kumada-cross-coupling-reaction-19-320.jpg)