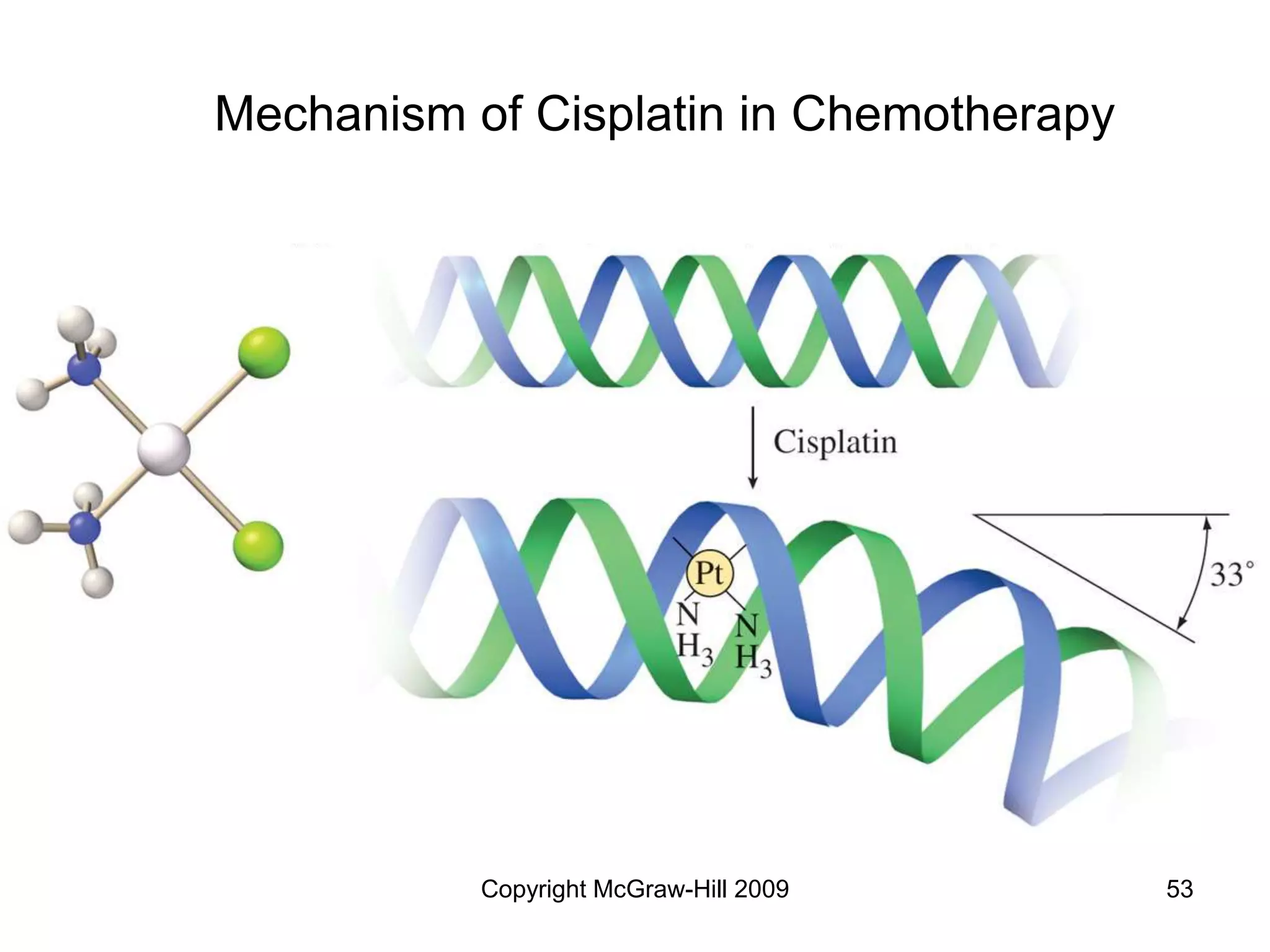
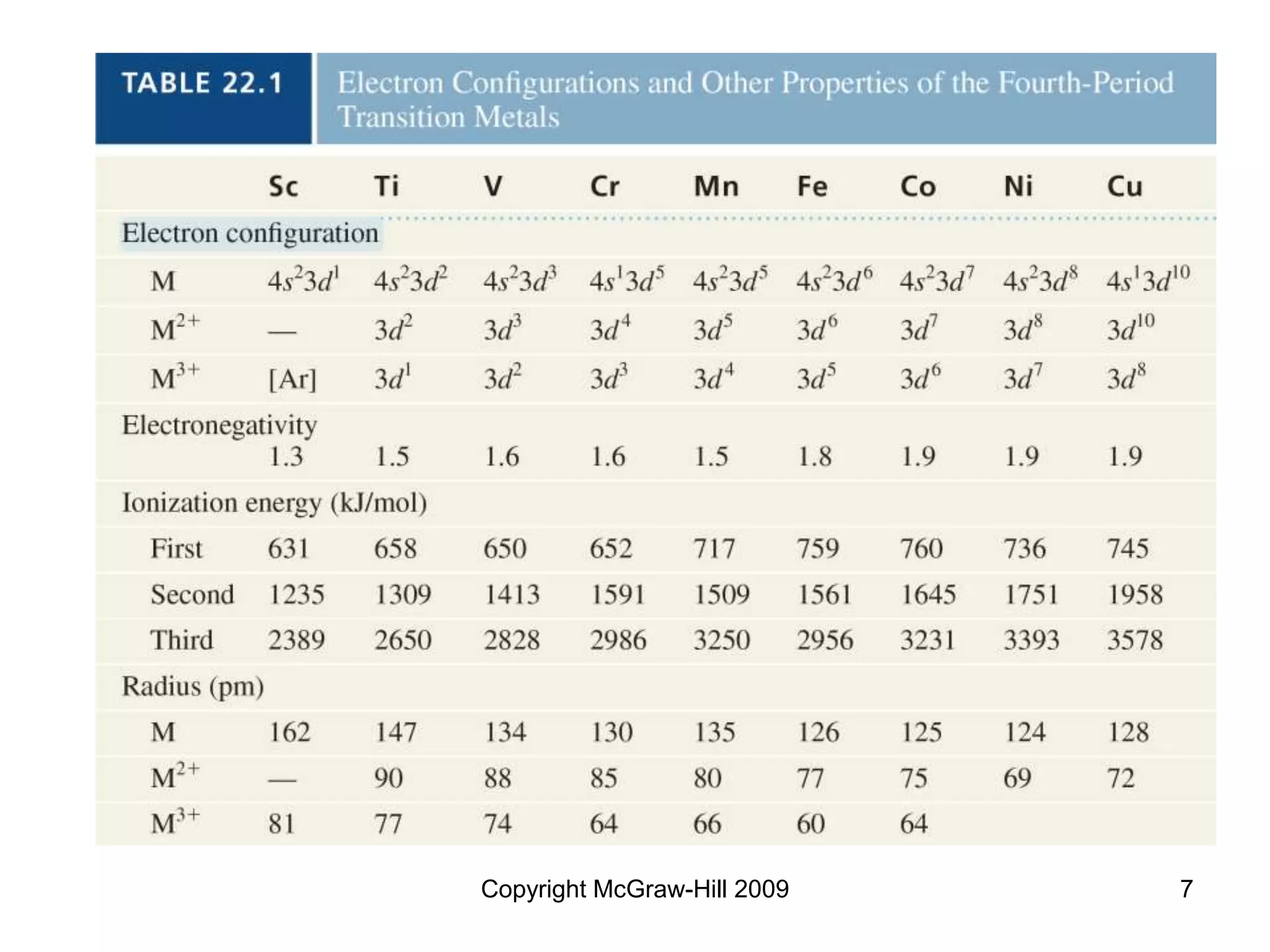
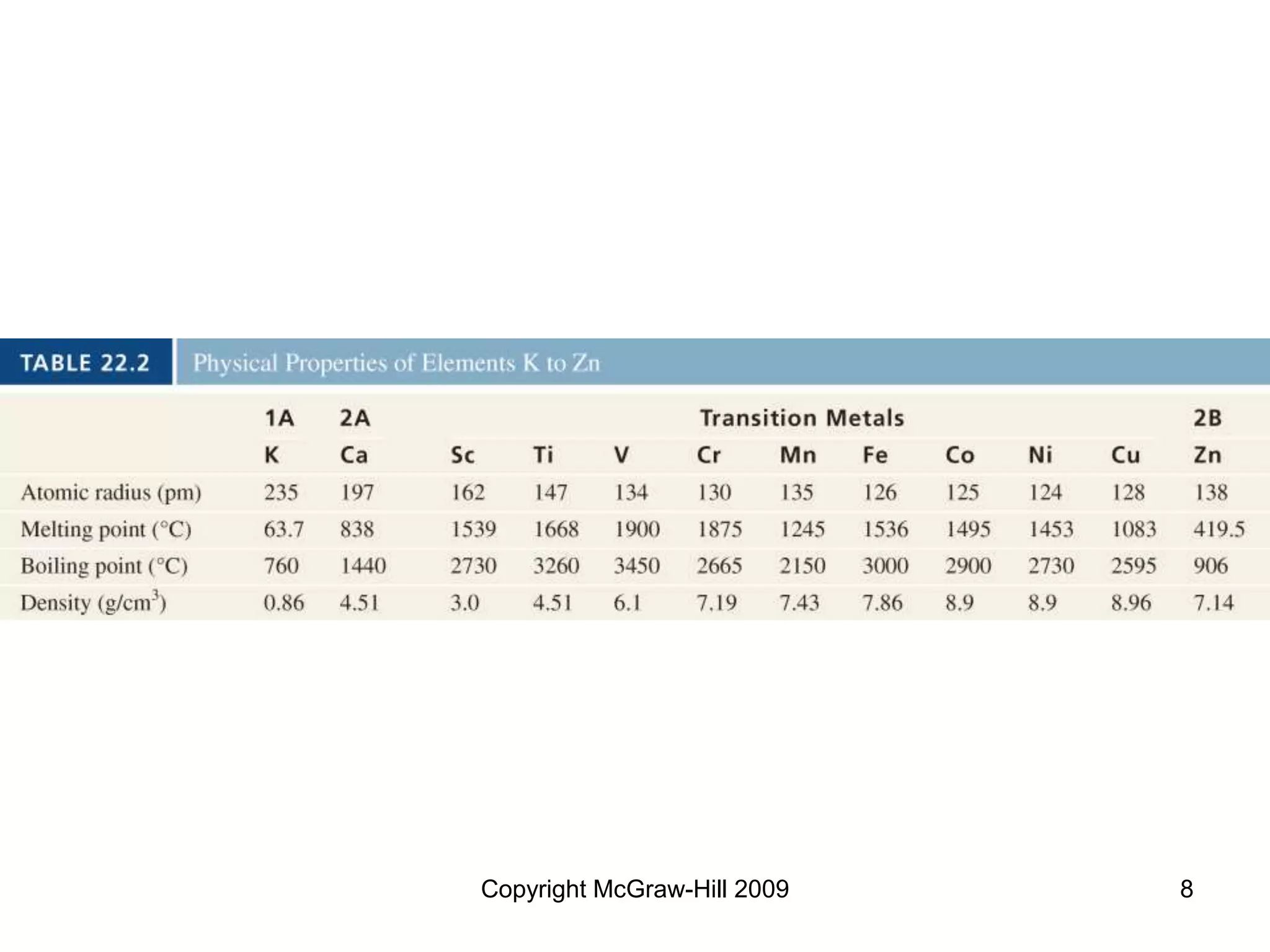
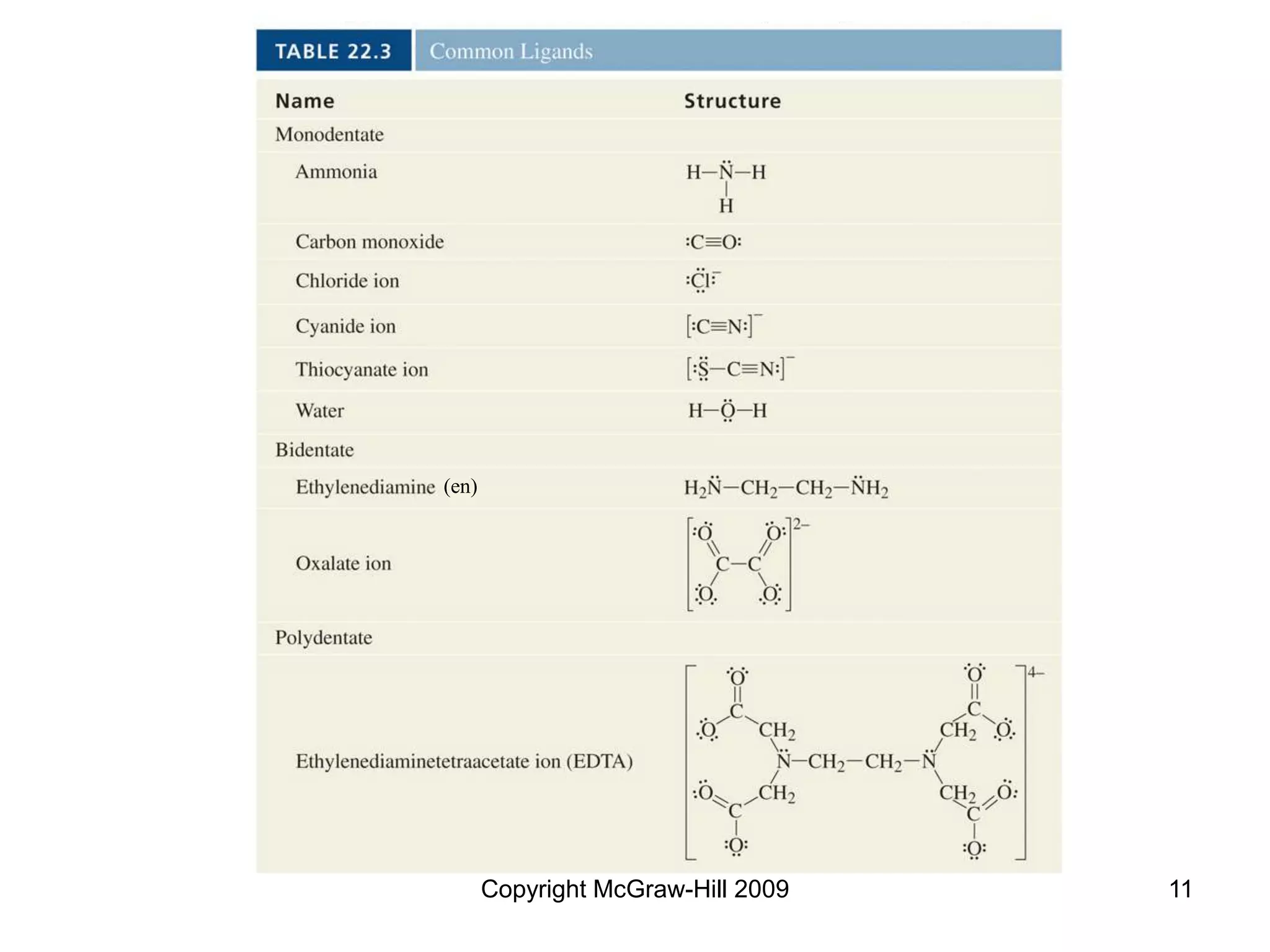
This document provides an overview of coordination chemistry and coordination compounds. It discusses the components and properties of coordination compounds, including ligands, complex ions, coordination numbers, and oxidation states. It also describes the structures of coordination compounds using crystal field theory and discusses the different geometries and resulting isomers. Finally, it covers reactions and applications of coordination compounds.








![Copyright McGraw-Hill 2009 12
Representations of [Co(en)3]2+](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-12-2048.jpg)
![Copyright McGraw-Hill 2009 13
Representations of [Pb(EDTA)]2](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-13-2048.jpg)

![Copyright McGraw-Hill 2009 15
Determine oxidation number for the transition
metal, Au, in
K[Au(OH)4]](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-15-2048.jpg)
![Copyright McGraw-Hill 2009 16
K[Au(OH)4] consists of a complex ion (the
part of the formula enclosed in square
brackets) and one K counter ion. Because
the overall charge on the compound is zero,
the complex ion is [Au(OH)4]. There are
four
ligands each with a 1 charge, making the
total negative charge 4. So the charge on
the gold ion must be +3.](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-16-2048.jpg)
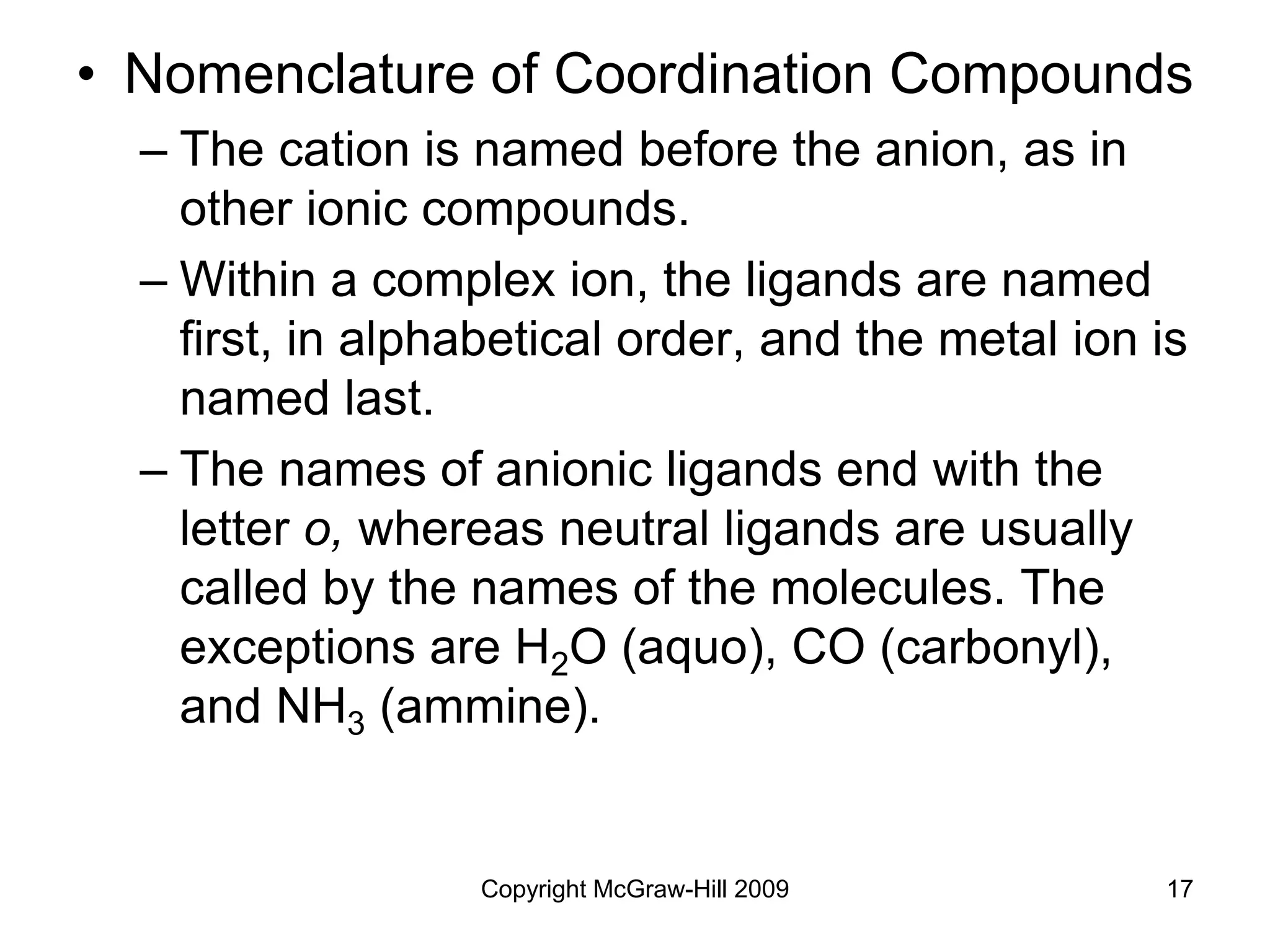

![Copyright McGraw-Hill 2009 19
Give the correct name for [Cr(H2O)4Cl2]Cl.](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-19-2048.jpg)
![Copyright McGraw-Hill 2009 20
Tetraaquodichlorochromium(III) chloride
[Cr(H2O)4Cl2]Cl](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-20-2048.jpg)

![Copyright McGraw-Hill 2009 22
[Co(en)3]2(SO4)3
tris(ethylenediamine)cobalt(III) sulfate](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-22-2048.jpg)















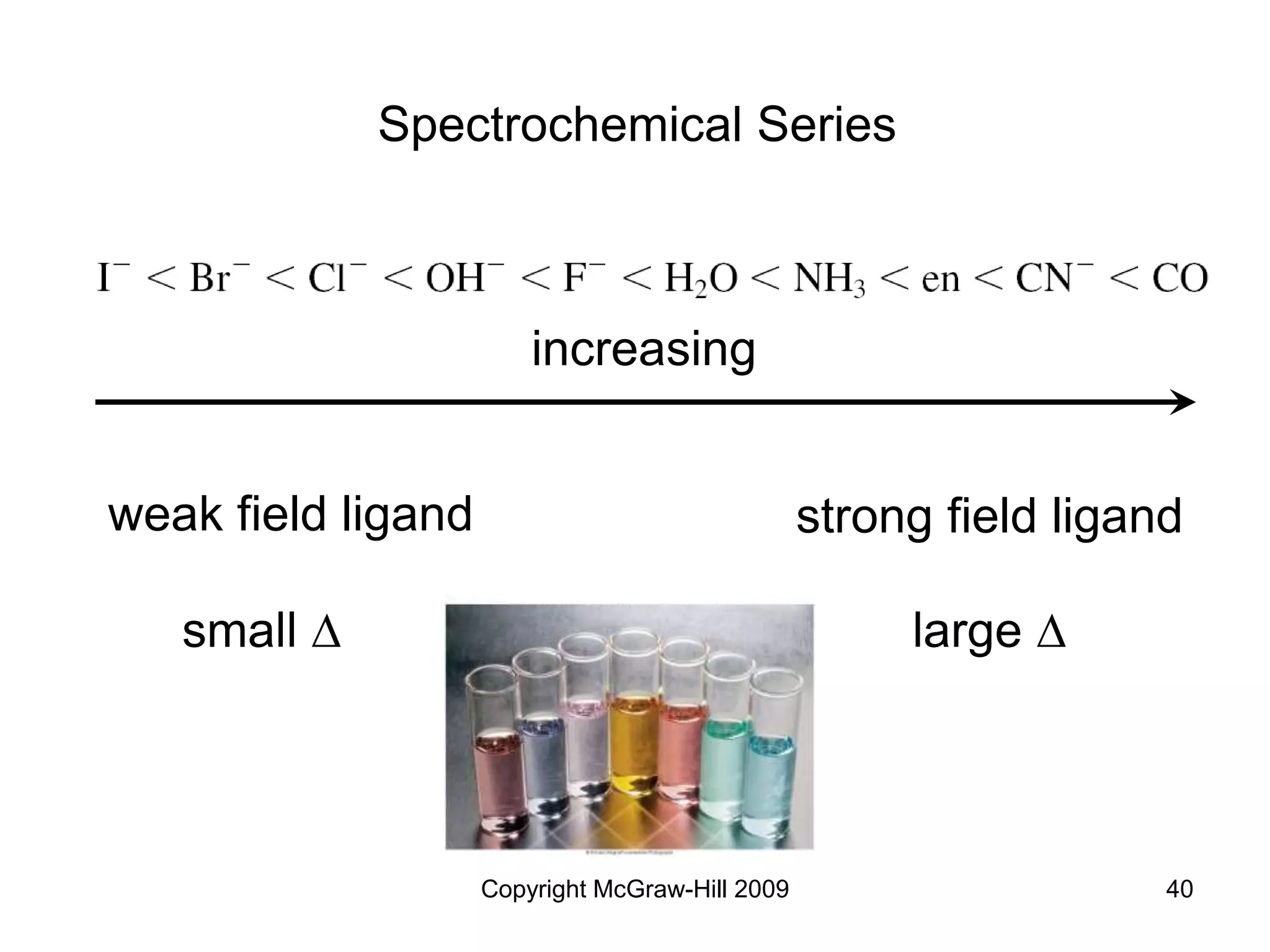




![Copyright McGraw-Hill 2009 48
How many unpaired electrons are in [Mn(H2O)6]2+?
Hint: H2O is a weak field ligand.](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-48-2048.jpg)
![Copyright McGraw-Hill 2009 49
Mn2+ has an electron configuration of
d5. Because H2O is a weak-field ligand, we
expect [Mn(H2O)6]2+ to be a high-spin
complex. All five electrons will be placed in
In separate orbitals before any pairing
occurs.There will be a total of five unpaired
spins.](https://image.slidesharecdn.com/4910457-221029082749-9df00778/75/4910457-ppt-49-2048.jpg)